(261c) High Throughput and Simple Fabrication of Compartmentalized Hydrogel Microbeads for Tissue Culture Applications
AIChE Annual Meeting
2022
2022 Annual Meeting
Materials Engineering and Sciences Division
Biomaterial Scaffolds for Tissue Engineering I
Tuesday, November 15, 2022 - 8:36am to 8:54am
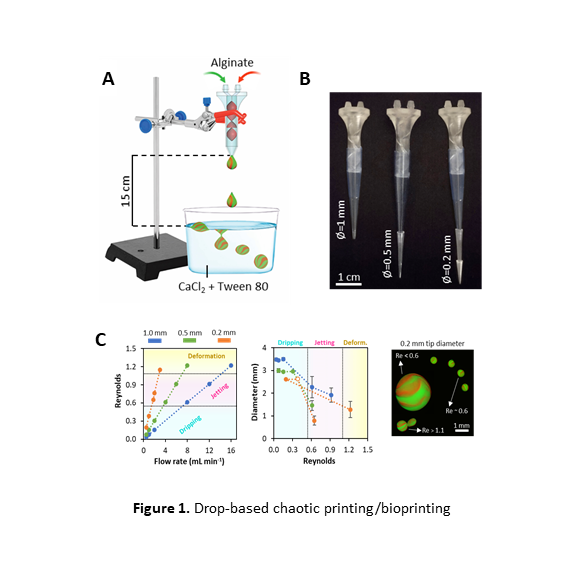
Here, we present a simple drop-based chaotic bioprinting method for the fabrication of microstructured and compartmentalized hydrogel microbeads for tissue engineering applications. We coextruded two alginate-based inks (or bioinks) through a printhead containing Kenics Static Mixing (KSM) elements and continuously produced drops with internal structure (i.e., alternating layers of two inks) by crosslinking in a calcium chloride/Tween 80 bath (Figure 1A-B). The number of KSM elements within the printhead determined the number of internal layers within the hydrogel beads, so that beads with 4, 8, 16, or 32 layers could be fabricated using printheads containing 2, 3, 4, or 5 KSM elements, respectively.
In a first set of experiments, we show that different drop sizes (from 500 to 3,500 µm) and shapes can be obtained by simply varying the flow rate (from 1 to 16 mL/min) and the diameter of the printhead tip (from 0.2 to 1.0 mm). Indeed, different regimes of fabrication can be clearly distinguished as a function of the Reynold’snumber of the system. As the flow rate is increased, the system transitions from a dripping (0< Re < 0.6) to a jetting (0.6 < Re < 1.0) regime, and spherical drops of smaller diameter are progressively obtained. At Re > 1.0, the system adopts a deformation regime and renders elongated particles (Figure 1C).
We explored the use of this fabrication strategy to engineer microstructured and compartmentalized spherical scaffolds for tissue engineering applications. For instance, we used inks or bioinks based on mixes of alginate and gelatin-methacryloyl (GelMA). In our experiments, we produced beads containing intercalated layers of mammalian cells (i.e., Caco2 cancer cells and fibroblasts), intercalated layers of mammalian cells and nanoparticles (i.e., C2C12 cells and bioglass, or intercalated layers of C2C12 cells and oil-water emulsions). We also demonstrated that the continuous culture of these microstructured beads in microfluidic chambers results in the fabrication of coherent microtissues (i.e., tumor-tissue models or pieces of cultured meat).
We envision that this simple drop-based fabrication technique that uses laminar chaotic flows will greatly facilitate the production of “living†beads for tissue engineering applications.