(196f) Structural Reorganization of Ionic Liquid Electrolytes By Charge/Discharge Circle
AIChE Annual Meeting
2022
2022 Annual Meeting
Innovations in Process Engineering
Ionic Liquids: Novel Separation, Catalytic reaction and Electrochemical Processes
Monday, November 14, 2022 - 4:40pm to 4:54pm
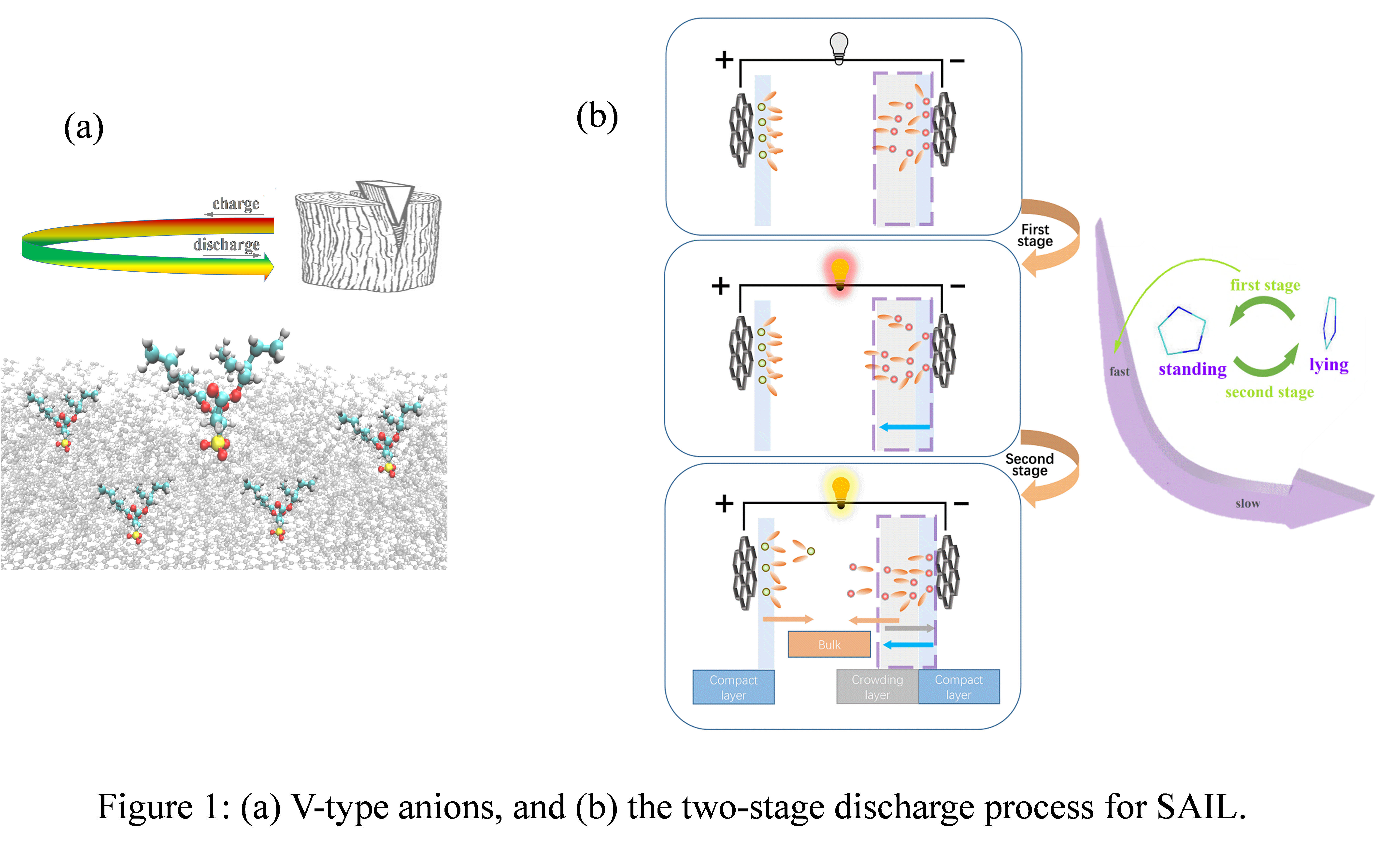
Recent experimental results showed that SAILs show better charge storage performance on charged surface than NAILs [2]. Near the positive electrode of [C4mim][AOT] system, it was found that [AOT]- would easily enter into the interfacial layer with a V-type conformation during the charging process by MD simulation. Compared with the simulated initial state, there were more V-type anions in the electrolyte after a process of charging and discharging, which is an evidence that the electrolyte was optimized. It provides an alternative way to optimize the IL electrolyte [3]. On the other hand, near the negative electrode of [Cnmim][AOT] and [Cnmim][BF4] systems, the structural transition of imidazolium-based NAILs and SAILs in a two-stage discharge process was discussed [4]. The two-stage discharge process of NAILs was more obviously, and it was slightly gentle for SAILs. It was revealed that the quick decrease in electric energy during the first stage of discharging was mainly due to the change of cations shapes near the negative electrode instead of their movement; the slow decrease in electric energy during the second stage of discharging was caused by overscreening and slow movement of cations from the negative electrode. Unlike the conventional view, it is concluded that the large scale movement of ions plays a relatively minor role in releasing electric energy. It will help us to understand the mechanism of energy release and provide a theoretical basis for the rational design of ILs-based electrolytes.
References
[1] Y. Wang, , H. He, , C. Wang, , Y. Lu, , K. Dong, , F. Huo, S. Zhang, Insights into Ionic Liquids: From Z-Bonds to Quasi-Liquids, JACS Au 2 (2022) 543-561.
[2] X. Mao, P. Brown, C. Cervinka, G. Hazell, T. A. Hatton, Self-assembled nanostructures in ionic liquids facilitate charge storage at electrified interfaces, Nature Materials 18 (2019) 1350-1357.
[3] K. Zhang, G. Zhou, T. Fang, K.Jiang, X. Liu, Structural Reorganization of Ionic Liquid Electrolyte by a Rapid Charge/Discharge Circle, Journal of Physical Chemistry Letters 12 (2021) 2273-2278.
[4] K. Zhang, G. Zhou, , T. Fang, X. Tang, X. Liu, Different shapes based on ionic liquid leading to a two-stage discharge process, Journal of Materials Chemistry A 10 (2022) 7684-7693.