(18f) Caffeine Removal Using "the Miracle Tree" Wastes for Sustainable Water Purification Processes As Alternative to Classic AOP and Commonly Used Filtration Media
AIChE Annual Meeting
2022
2022 Annual Meeting
Environmental Division
Fundamentals of Food, Energy, Water Systems
Sunday, November 13, 2022 - 5:35pm to 6:00pm
Two sets of batch experiments were performed. Set one involved the treatment of synthetic wastewater samples at a starting initial concentration of 100 ppm subjected to following processes: Fenton and photo-Fenton, photocatalysis using TiO2, and coagulation/floculation using moringa oleifera cotyledon (MOC) extracts along with NaCl (MOC1-NaCl). The second set of experiments explored the purification of caffeine solutions at lower initial concentrations (5, 25 and 50 ppm), which were treated through a modified Fenton process (an optimized version) and MOC with NaCl (MOC2-NaCl) with slight variations from the 100 ppm experiments. MOC2-NaCl plus caffeine experiments were designed in order to reflect a more realistic situation in terms of the abundance of caffeine in one of the most important discharges of Quito as well as to propose dosages (in mL) of the MO extracts into the caffeine samples which provides a more simple and practical way compared to MOC1-NaCl and caffeine experiments.
It was found that the larger the amount of Fe(II), the higher the degradation of caffeine. However, when 0.18 mmol/L and 0.36mmol/L of ferrous sulphate is used similar caffeine degradation is observed. Photo-Fenton produces a higher caffeine degradation (99%) when compared to Fenton (66%) at the same Fe(II) concentration (0.36mmol/L). A lower caffeine degradation (53%) was observed using TiO2/UV and TiO2 process conditions could be optimized in the future to yield to an even higher caffeine degradation, comparable to the one observed for Photo-Fenton.
We found that a practical way to remove organic contaminants, although through a very different process than the one observed in Fenton and Photo-Fenton methods, is the use of Moringa Oleifera seeds extracts. These seeds are known to have flocculant functionalities . Therefore, caffeine is not degraded but rather removed through adsorption and coagulation-flocculation mechanisms by the seeds extract. This process does not involve the use of any chemicals nor light chambers, and constitutes a simple procedure that can be easily applied and extended to other compounds. A dosage between 3-10 mL of MOC-NaCl is recommended to naturally achieve 61.28 ± 0.021% (5 mL) removal after one hour of process. Caffeine degradation by Fenton reaction occurs mainly within the first 5 to 10 min (67.35 ± 0.043%).
Borosilicate columns with 11 mm inner diameter and 60 cm height were used for filtration experiments. They were cleaned up by adding a 0.1 M NaOH solution until filled. Subsequently, they were washed with distilled water so that a final solution of pure ethanol could be introduced to fill up the column. This was left for 2 minutes before it was released. Finally, the empty columns were left to dry overnight so that any solvent left inside the column could evaporate. When packing the columns with the filtration beds, each bed (except for Moringa oleifera husk) was carefully washed with water until all possible contaminants (such as dirt or small rocks) were carried away. Then, they were left to dry out at room temperature for 3 hours. Afterwards, each bed was placed in the borosilicate column up to the desired depth; a gentle shake was also given to ensure a better packing. Finally, 100 mL of water were passed through the column with thebed to guarantee that the fluid pathway was also cleaned. In the case of Moringa oleifera husk, a different approach was taken. Firstly, the Moringa oleifera husk was cleaned, washed and sieved for 1 minute. A small quantity of this husk was then added to the column entrance and gently pushed to the bottom of the column with the help of a rod, always checking that the husk did not block the pathway. The procedure was repeated for the rest of the husk until the column was packed with the desired depth.
Five different models were tested on each breakthrough curve for every filtration bed. The parameters and variables for every model were optimized for each case in order to obtain a best fit model. A direct measure of the efficiency of filtration media may be achieved by plotting the percentage of caffeine removal as a function of time for a fixed bed depth. The ability to retain caffeine will then decrease over time. It can be observed that the bed that maintains the highest percentage caffeine removal through operation time is zeolite. However, based on the models that best describe experimental data it can be inferred that zeolite does not produce the highest percentage of caffeine removal at the beginning of the operation. When the first drop of the effluent appears (when t = 0), the percentage of caffeine removal of zeolite is barely 75% while other beds such as Moringa oleifera husk and gravel present a percentage removal of 96% and 81% respectively. From this perspective, zeolite will be preferred as the filtration medium if the priority is to maintain a high percentage removal through time. However, other beds such as the Moringa oleifera husk or gravel will be preferred if what is sought is to obtain an instantaneous caffeine removal at the beginning of the operation. It was found that in average, the unit mass of zeolite adsorbed 5.640 × 10-7 ± 1.007 × 10-7 g of caffeine.
The selection of "best filtration bed" will depend on what variables are considered a priority. For instance, if the main goal is to achieve a maximum removal with a fixed bed depth, zeolite will be chosen. However, if the purpose is to obtain the most pollutant retention with the least amount (mass) of medium, then the Moringa oleifera husk will be the best filtration bed. Conversely, if there is an economic analysis on which is the cheapest and the most abundant bed that could be used, then gravel will appear as the best candidate. This section was specifically focused on the dynamics of pollutant removal; nonetheless, a hydraulic analysis may be recommended for determining the possibilities and limitations of a real system. Sometimes the effectiveness of the medium will not only be determined by the pollutant removal, but also by the amount of fluid it can treat per unit time. Henceforth, hydraulic head loss in conjunction with the analysis done in this investigation will ultimately indicate which bed is the best for treating a specific fluid flow. It is important to notice that even though the characterization of filtrarion media were done with their depth-mass relation, it is not the mass per se that is responsible for adsorption; it is its superficial area. To consider a possible scale up, an increase of the surface area available should be achieved in the system for the breaking point to occur at time of 1 hour with a fluid flow of approximately 1 mL/min. It is recommended to enhance and develop a relationship between filtration bed mass to its superficial area. Varying the superficial area of the beds through particle size could also be explored to determine which combination yields the best percentage of pollutant removal considering hydraulic head losses. A combination of these beds may result in the optimum dynamic filtration system if scale-up is desired.
The use of MO lignocellulosic wastes from agroindustries reduces the negative impact of these wastes in the environment and allows and efficient and sustainable use of a natural renewable resource, which otherwise does not enter in the value chain of the agroindustry production; and in the other hand minimizes the emergent pollutants in contaminated water, making it safer for consumption. In this way, being focused on sustainability, carbon foot print may be reduced and communities may live in healthier living conditions.
References
Landázuri, A. C., Villarreal, J. S., Andrade, J. C., Sornoza, I., & Lagos, A. S. (2019). Bulk balance filtration model (BBFM) for lead and iron physisorption through Moringa oleifera Lam. seed husks. Journal of Environmental Chemical Engineering, 7(5), 103302.
Landázuri, A. C., Paz, R., Álvarez, A., Alina Trávez, Arleth Gualle, Juan S. Villarreal, Javier Gándara, Andrés Lagos, Verónica Castañeda, Emilia Morales, Javier Garrido, Gabriela Vernaza, Lucía Ramírez, Danny Navarrete, Lorena Bejarano, Caicedo, A., & Orejuela, L. (2020). All for one and one for all research: Moringa oleifera Lam. in food, health and environmental applications. AIChE Annual Meeting, Conference Proceedings.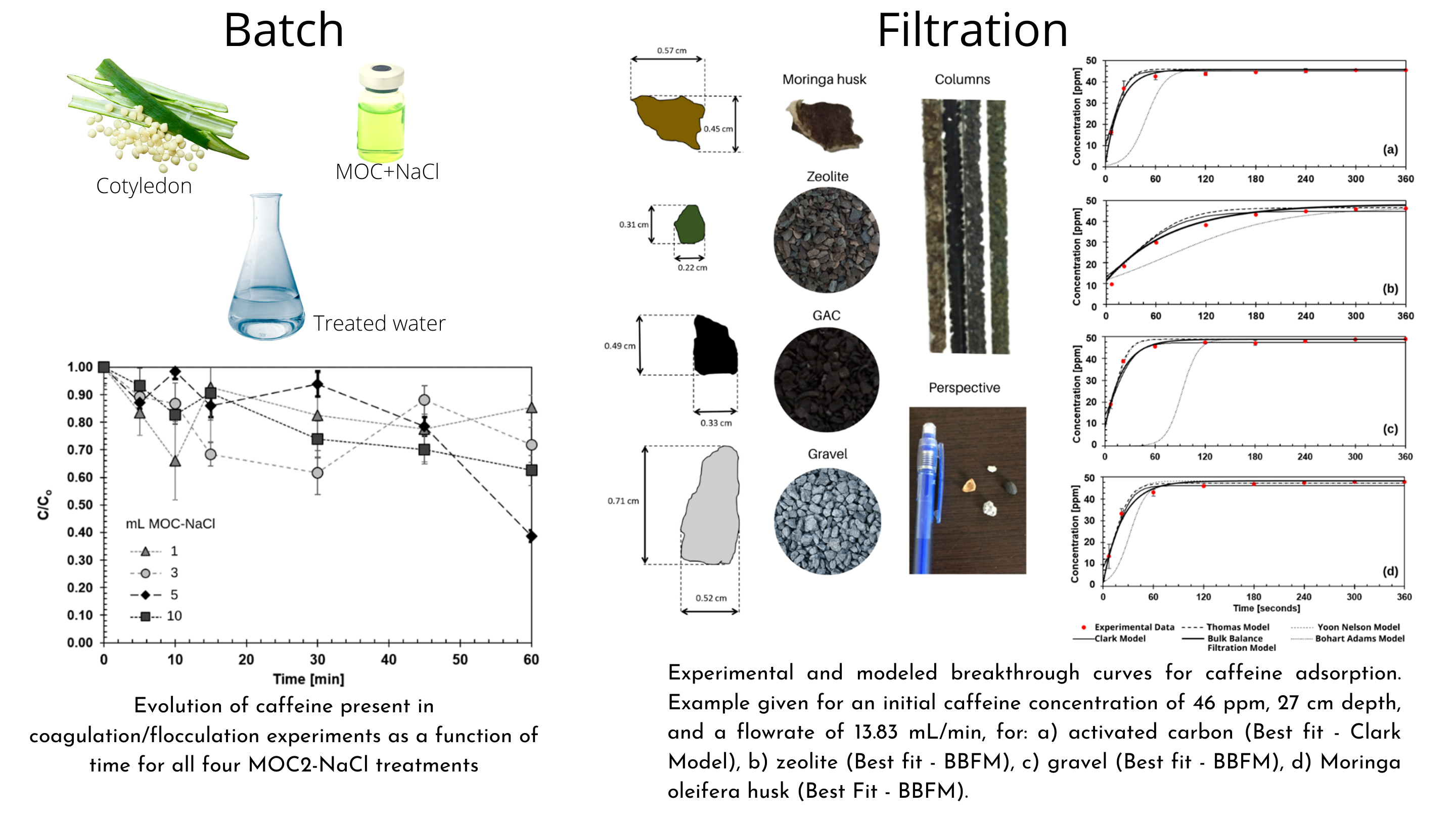
Checkout
This paper has an Extended Abstract file available; you must purchase the conference proceedings to access it.
Do you already own this?
Log In for instructions on accessing this content.
Pricing
Individuals
| AIChE Pro Members | $150.00 |
| AIChE Emeritus Members | $105.00 |
| AIChE Graduate Student Members | Free |
| AIChE Undergraduate Student Members | Free |
| AIChE Explorer Members | $225.00 |
| Non-Members | $225.00 |
