(101a) Effects of Lewis Acidity and Confinement on Aldol Reactions of Aldehydes in Zeotypes
AIChE Annual Meeting
2022
2022 Annual Meeting
Catalysis and Reaction Engineering Division
Microporous and Mesoporous Materials III: Structure
Monday, November 14, 2022 - 12:30pm to 12:48pm
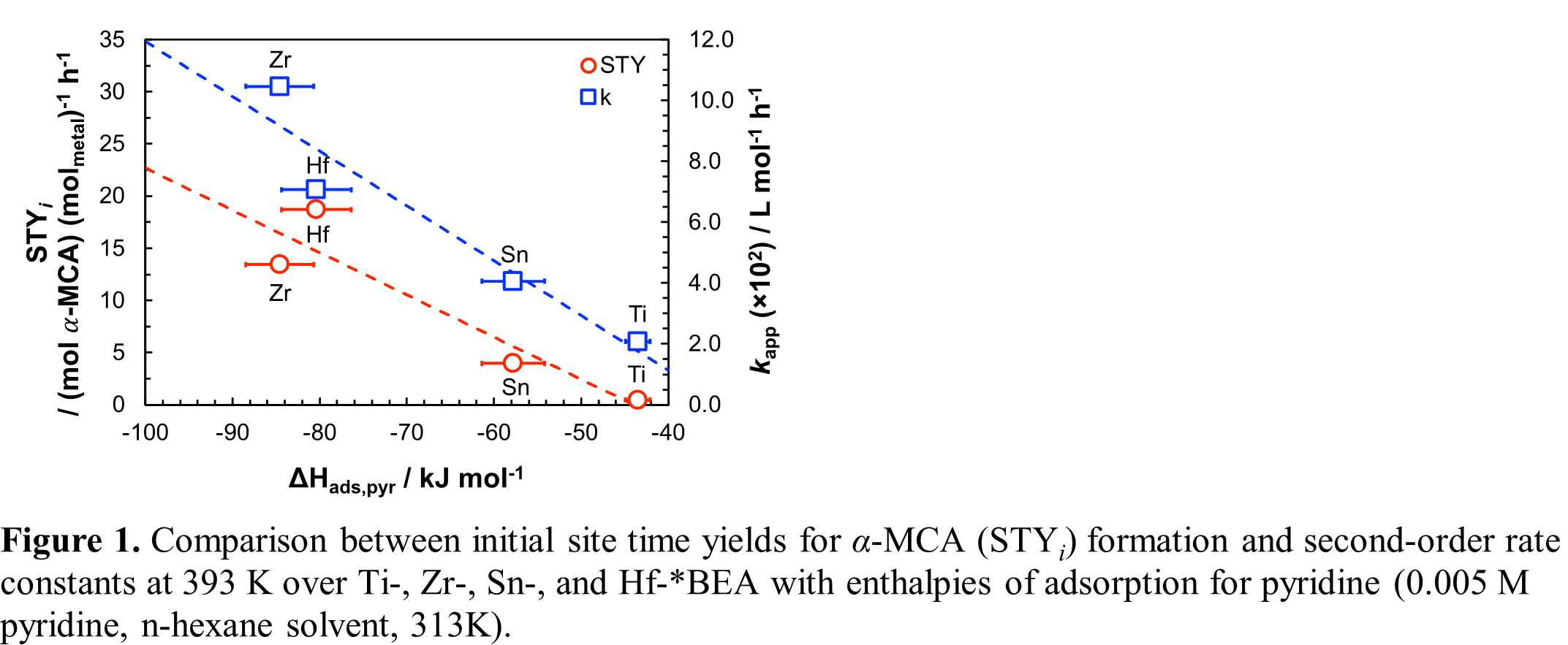
Here, the Claisen-Schmidt condensation of benzaldehyde and propionaldehyde was used as a probe reaction to examine the intrinsic activity of Ti-, Zr-, Sn-, and Hf-containing zeotypes by considering the influence of Lewis acidity along with the confining reaction environment on apparent rate constants, product site time yields (STY), and activation barriers. Experimental evidence revealed that initial rates of enolization at 393 K follow a pseudo-second-order dependence over large-pore *BEA (~7 Ã…) zeotypes with differences in STYs for α-methyl cinnamaldehyde (α-MCA), the cross-condensation product, that correlate with measured adsorption enthalpies for pyridine (Zr > Hf > Sn > Ti) as a proxy of functional Lewis acid strength (Figure 1). When Lewis sites are confined within medium‑pore MFI (~6 Ã…) zeotypes, initial rates of Âcross-aldol condensation exceed those for self‑condensation of propionaldehyde, indicating that selection of pore size and benzaldehyde/propionaldehyde ratios can be used to steer product selectivity.