(100a) Irreversible Adsorbate Thermodynamics on Zeolite Surfaces
AIChE Annual Meeting
2022
2022 Annual Meeting
Catalysis and Reaction Engineering Division
Fundamentals of Catalysis and Surface Science II: Zeolites and acid catalysis
Monday, November 14, 2022 - 12:30pm to 12:48pm
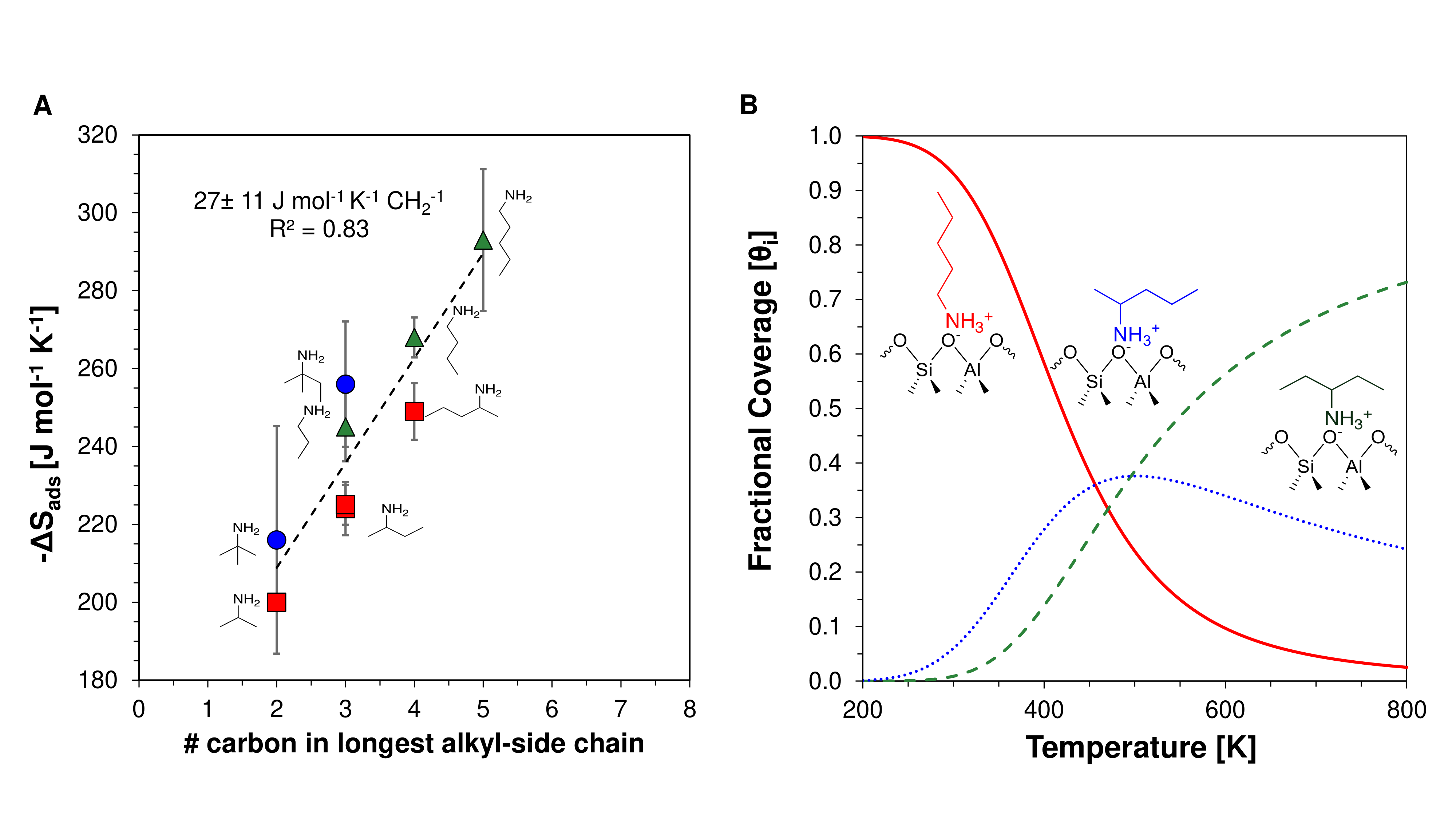
In designing solid acid catalysts, understanding adsorption energetics informs catalyst optimization for different chemistries, where the relative stability of surface species dictates the overall catalytic turnover frequency. While enthalpic contributions are relatively well understood, limited information exists for entropic contributions to adsorption, particularly chemisorbed species, representative of catalytically relevant reaction intermediates and transition states, especially suffer from the lack of entropic understanding. The challenge in measuring the entropy of a chemisorbed species results from existing experimental methods requirement of establishing adsorption/desorption equilibrium.Strongly bound intermediates like alkylamines adsorbed on a Brønsted acid site, however, equilibrate on infinitely long timescales, limiting the applicability of existing experimental techniques. Here, we leverage the concept of adsorption assisted desorption through the co-adsorption of multiple strongly bound adsorbates to attain adsorption equilibrium. Applying this concept to alkylamine adsorption on Brønsted acid sites in H-MFI, we demonstrate for the first time the experimental ability to measure the overall adsorption thermodynamics of strongly bound species. Comparing a homologous family of alkylamines (C3-C5), we find a fixed contribution to both the enthalpy (17 kJ mol-1 CH2-1) and entropy (27 J mol-1 K-1 CH2-1) of adsorption per methylene unit on isolated sites (Si/Al=140). The preference for terminal adsorption of n-alkylamines when compared with the central adsorption of sec-alkylamines, changes at temperatures > 460 K. From the interaction of multiple alkylamines, with different carbon number, we find a simple descriptor based on the longest alkyl-side chain for alkylamine energetics at equilibrium (Figure 1A). Adsorption selectivity as determined by these studies, inform a change in preference of orientation along a C5 backbone, with catalytic temperature (Figure 1B). Ultimately, the adsorption interactions of multiple alkylamines on various zeolite frameworks, provide the means to compare, select and optimize zeolite catalysts for different chemistries based on adsorbate energetics of transition-state-like molecules.