A new approach to cell therapy deploys engineered cells from the human gut microbiome to treat disease.
Remarkable progress in biomedical therapeutics has been achieved through the successive emergence of novel treatment modalities, with each new class of drugs opening up an exciting world of possibilities to address the diversity of human disease. In the 1980s and 90s, “biologics” came onto the pharmaceutical scene as an exciting new alternative to traditional small molecule drugs. To name just a few, biosynthetic insulin and antibody-based therapies, such as Humira and Herceptin, altogether revolutionized medical care for patients with metabolic disease, inflammatory conditions, and cancer. Similarly, the past two decades have followed with the invention of even more complex so-called “cell therapies,” wherein patients are transplanted with functional, living human cells to treat a disease. For example, in CAR T-cell therapy, a patient’s immune cells are removed from the blood, propagated, and genetically engineered in the laboratory to enhance their activity, and then transplanted back into the patient to fight against cancer. Such innovations have revolutionized the field of oncology and are now considered standard of care for a variety of blood cancers (1).
At Novome Biotechnologies, we believe that cell therapies are the future of biomedical innovation. We are pushing the envelope for this class of drugs by engineering bacterial cells, not human cells, to transplant into patients. These bacterial cells live in the gut while treating disease and serve as the first engrafting, engineered microbial cell therapies to be developed.
Why use bacteria? The human colon is home to trillions of diverse, commensal (symbiotic) bacterial cells, and as such is arguably the most meaningful natural interface between the human body and foreign genetic material (Figure 1) (2). This incredibly rich and dense bacterial community (i.e., our microbiota) has a central — if still poorly understood — role in promoting health and disease outcomes. No two human microbiotas are exactly the same, and the activity of this complex internal ecosystem has so many important impacts on human health that some have referred to it as a “supporting organ.” Despite the clear importance of our microbiota in shaping whether we develop a given disease or respond to a particular drug therapy, the inherent complexity of this internal ecosystem has both obscured the biological details of how it functions and hindered attempts to selectively modify it.
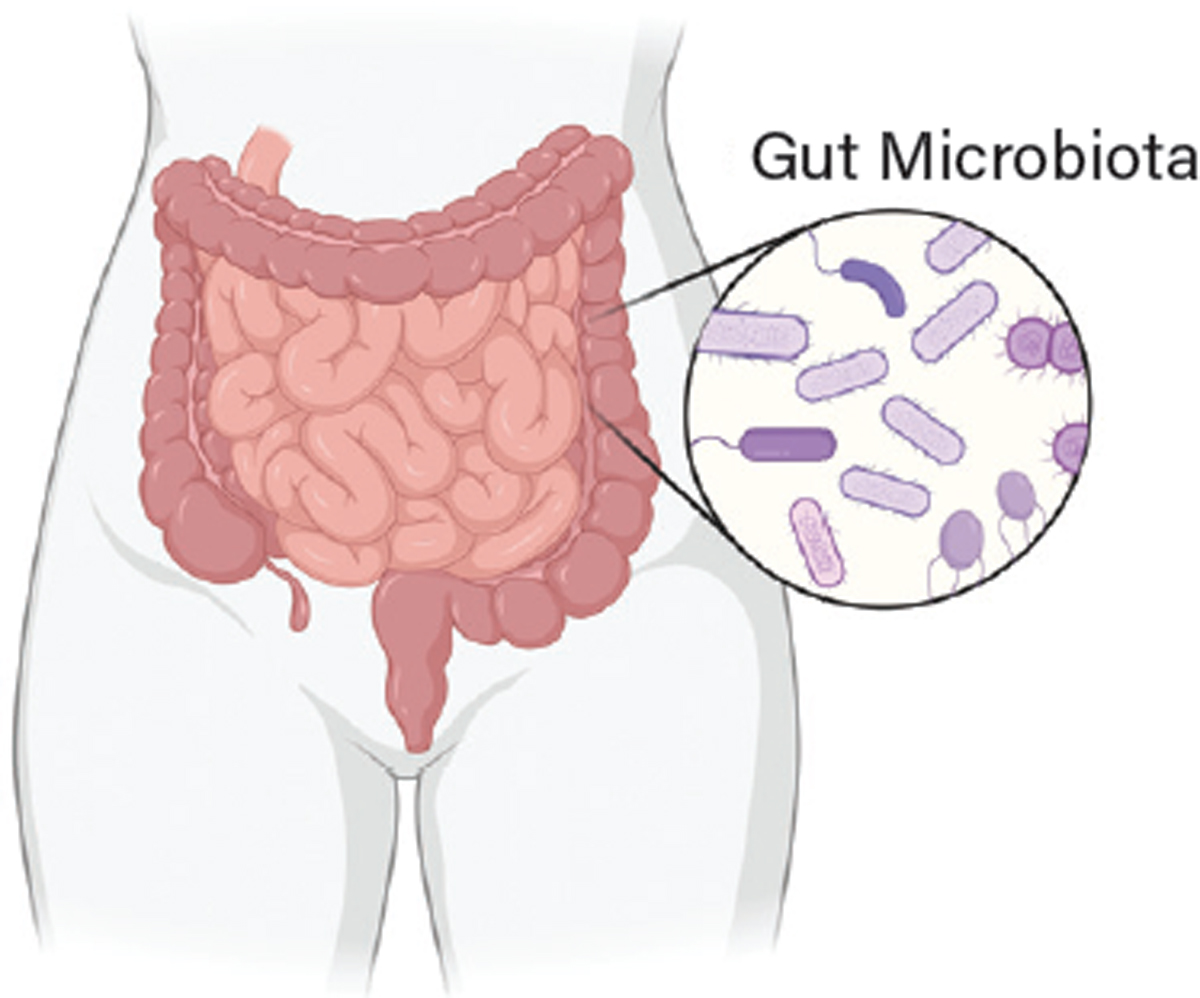
▲Figure 1. The human large intestine is home to trillions of diverse microbial residents: predominantly bacteria but also fungi, parasites, and viruses. The sum total of this community is dubbed the “gut microbiota.” The majority of these microbial residents are symbiotic, meaning both the bacteria and the human body benefit from their presence. Because the human gut microbiota factors into many diseases — impacting the gastrointestinal system and beyond — it is an ideal location to deploy engineered microbial cell therapies.
Some companies, looking to leverage the power of our gut microbes as therapeutics, have adopted a holistic approach of transplanting entire naturally occurring communities from healthy individuals into patients with a particular disease, in a process called fecal microbiota transplant. Such donor-derived microbial therapies are inherently variable and attempt to promote health via largely unknown molecular mechanisms. Furthermore, introducing a new gut ecosystem wholesale requires initially displacing the patient’s native microbiota with the use of broadly acting antibiotics, and even with such dramatic perturbations, the transplanted community may not persist over time (3).
In contrast, at Novome, we are applying synthetic biology tools to engineer defined therapeutic activities into a single gut commensal bacterial genus, Bacteroides. We introduce our genetically engineered microbial medicines (GEMMs) into patients as a unique cellular therapy: a single bacterial strain that is rationally designed to engraft (or “colonize”) into the gut and deliver its health-promoting effects with defined, controllable, and reversible activity. There are currently no FDA-approved engineered live bacterial therapeutics, and we are working to bring our vision of this new class of drugs to patients.
This article describes Novome Biotechnologies’ approach to making engineered microbial cell therapies, including the key challenges and our solutions, real world proof of concept of this approach in ongoing clinical trials, and how we are expanding our platform to work in big disease spaces like inflammatory bowel disease (IBD)...
Would you like to access the complete CEP Article?
No problem. You just have to complete the following steps.
You have completed 0 of 2 steps.
-
Log in
You must be logged in to view this content. Log in now.
-
AIChE Membership
You must be an AIChE member to view this article. Join now.
Copyright Permissions
Would you like to reuse content from CEP Magazine? It’s easy to request permission to reuse content. Simply click here to connect instantly to licensing services, where you can choose from a list of options regarding how you would like to reuse the desired content and complete the transaction.
