Chemical engineers at the University of Wisconsin-Madison have used a combination of advanced computation methods to create a more complete picture of the complex catalytic chemistry in fuel cells. The advance is an important step towards cost-effective fuel cells, which now rely on expensive platinum catalysts.
Eliminating platinum
Fuel cells generate electricity by combining electrons and protons, from a chemical fuel such as methanol, with oxygen from the air. To quicken the reaction, a catalyst comes into play. But the costly catalyst platinum is largely used today as the result of trial and error, since the complex catalytic chemistry involved is not well understood. Manos Mavrikakis, a UW-Madison professor of chemical and biological engineering, explains that being able to replace expensive platinum catalysts first relies on understanding the reaction mechanisms involved.
More complex than it appears
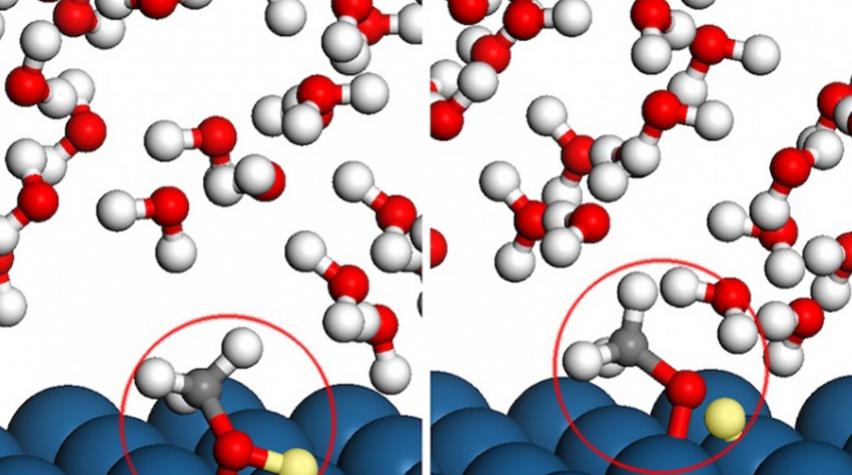
Briefly put, methanol molecules bathed in a watery environment settle on a platinum surface, giving up one of their four hydrogen atoms. The release of these atoms creates an electric current. While this seems simple, the actual process is far more complex.
The water and methanol molecules dance about the catalyst surface and fluctuate continuously. And not only do the methanol molecules react with the platinum, but occasionally so do the water molecules. In addition, varying voltage on the electrified surface of the platinum catalyst adds to the complexity. To better understand what occurs during this reaction, the researchers turned to computational simulations.
Computational techniques shed light
The inquiry began with the application of density functional theory to solve for quantum mechanical forces and energies between individual atoms. The researchers then built a scheme upon those results using molecular dynamics methods to simulate large ensembles of water and methanol molecules interacting among themselves and with the platinum surface.
The detailed simulations revealed that the presence of water in a fuel cell plays a huge role in dictating which hydrogen atom breaks free from methanol first — a result that simpler methods could never have captured. Electric charge also determined the order in which methanol breaks down, surprisingly switching the preferred first step at the positive electrode.
To learn more about this work, see the news release as well as the researchers' published work.


