

2011 Spring Meeting
One of the more interesting concepts in dealing with waste materials is the desire to convert them to higher value materials for reuse or as feed stocks for existing chemical and pharmaceutical processes. Due to the large volume of cellulosic-based wastes, the pyrolysis of cellulose to levoglucosan and other anhydrosaccharides (AS) is a current area of study for a research group at the Institute of Chemical and Engineering Sciences (ICES) in Singapore.
The Research
Review of the research indicated two pyrolysis pathways: transglycosilation and ?-elimination with intermediates cross-feeding the alternate reaction pathways. As the different pathways yield different end products, the ability to catalyze the desired path (to the desired products) is of key importance. Some interesting (and conflicting) findings on the effects of various acids (sulfuric, phosphoric, and boric) and alkalis (ammonium hydroxide, calcium hydroxide, and sodium hydroxide) on the production of AS was the impetus for further study. The apparatus used to conduct the reactions was a simple 1" stainless steel tubular reactor in a furnace, with reaction products cooling, condensing (liquids), and gas collection for analysis via GC/MS.
Results obtained confirmed that acids suppress the formation of AS at all temperatures, with higher concentrations resulting in greater
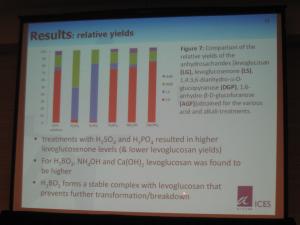
suppression. Sulfuric acid was the worst "offender." Phosphoric acid was less detrimental to AS formation, and boric acid had only a moderately negative effect, and one that was similar for all concentrations tested.
The results for the alkalis were the opposite of that seen for the acids. Additions of small amounts increased the production of AS (over non-catalyzed conditions), with the effect becoming much more significant as the temperature increased. Ammonium hydroxide had the most significant and predictable effect, whereas the results for calcium hydroxide were mixed. The current postulated reason for this alkali-catalyzing effect is the effect of suppression of volatile acid byproducts of the pyrolysis reactions.
Thus, for the preferential utilization of the transglycosilation pathway, efforts should focus on the alkali-catalyzation effect.
Next Steps
The group plans to continue the evaluation of various catalytic effects in an attempt to optimize conditions for the highest yields of the different target products. In addition, more work needs to be done using "real world" cellulose sources, and developing strategies to deal with the problems inherent in their use--especially the "ash" problem.
It was noted in the introduction of the presentation that the U.S. DOE is currently, or planning, to fund billions of dollars of research in the area of waste conversions in the biomass, municipal solid waste, and waste oils areas, among many others.

For More Information
Contact Salim Shaik via e-mail at shaik_salim@ices.a-star.edu.sg


