
Pyromaniacs at UMass Amherst have been on fire lately. In January, George Huber, associate professor of chemical engineering, using non-food biomass, a new catalyst and fast pyrolysis, boosted the yield for five key chemical industry building blocks by 40 percent. Queuing up quickly in February, Paul Dauenhauer, a prolific UMass chemical engineering enfant terrible, announced a breakthrough studying a small surrogate molecule that facilitated biomass pyrolysis computer simulations.
Dauenhauer's latest pyrolysis advance (press release), obviously multitasking this winter in the lab: finding an efficient, renewable way to produce the common plastic p-xylene, currently used in many products including soda bottles and food packaging. He says that while the plastics industry currently produces p-xylene from petroleum, his new biomass process creates exactly the same chemical. The research is published in the journal ACS Catalysis.
You can mix our renewable chemical with the petroleum-based material and the consumer would not be able to tell the difference," Dauenhauer said in the press release.
A specifically designed catalyst
The new process uses a zeolite catalyst capable of transforming glucose into p-xylene in a three-step reaction within a high-temperature biomass reactor. Dauenhauer says this is a major breakthrough since other methods of producing renewable p-xylene are either expensive (e.g., fermentation) or are inefficient due to low yields.
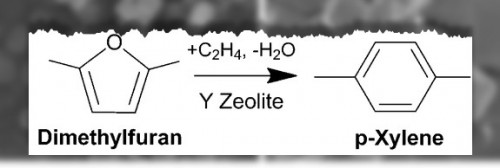
A key to the success of this new process is the use of a catalyst that is specifically designed to promote the p-xylene reaction over other less desirable reactions. Dauenhauer says his research colleagues, professors Wei Fan of UMass Amherst and Raul Lobo of the University

of Delaware, designed the catalyst. After a series of modifications, the team was able to help enhance the yield of the reaction.

Computations conducted by the team have been instrumental in understanding the reaction mechanism and the role of the catalyst as well as in altering the catalyst to improve yield. The research team discovered that the performance of the biomass reaction was strongly affected by the nanostructure of the catalyst, which they were able to optimize to achieve yields of 75%.


