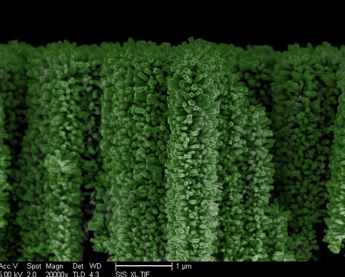
University of California, San Diego electrical engineers have managed to build a forest of tiny nanowire trees to capture solar energy and then use it for hydrogen fuel generation.
Reporting in the journal Nanoscale, the team said the nanowires, which are made from abundant materials like silicon and zinc oxide, are also a cheap way to deliver hydrogen fuel on a mass scale.
"This is a clean way to generate clean fuel," said Deli Wang, professor in the Department of Electrical and Computer Engineering at the UC San Diego Jacobs School of Engineering. (UCSD press release)
The trees' vertical and branch structures are keys to capturing the maximum amount of solar energy, according to Wang. That's because the natural structure of trees grabs and adsorbs light while flat surfaces simply reflect it, Wang said, adding that it is also similar to retinal photoreceptor cells in the human eye.
Water-splitting and hydrogen
Wang's team has mimicked this structure in their "3D branched nanowire array," which then uses photoelectrochemical water-splitting to produce hydrogen gas. This process uses clean energy with no green-house gas byproduct. By comparison, the current conventional way of producing hydrogen relies on electricity from fossil fuels.
"Hydrogen is considered to be clean compared to fossil fuels because there is no carbon emission, but the hydrogen currently used is not generated cleanly," said Ke Sun, a PhD student in electrical engineering who led the project. Also, by harvesting more sun light using the vertical nanotree structure, Wang's team has developed a way to produce more hydrogen fuel efficiently compared to planar counterparts.
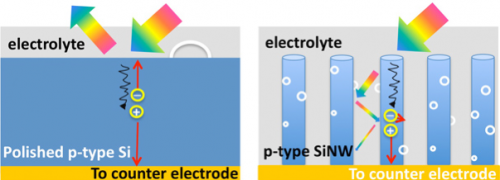
The vertical branch structure also maximizes hydrogen gas output, said Sun. For example, on the flat wide surface of a pot of boiling water, bubbles must become large to come to the surface. In the nanotree structure, very small gas bubbles of hydrogen can be extracted much faster. "Moreover, with this structure, we have enhanced, by at least 400,000 times, the surface area for chemical reactions," said Sun.
Biomimicry and hydrocarbons
In the long run, what Wang's team is aiming for is even bigger: photosynthesis. Wang's team hopes to mimic this process and capture CO2 from the atmosphere, reducing carbon emissions, and convert it into hydrocarbon fuel.
The team is also studying alternatives to zinc oxide, which absorbs the sun's ultraviolet light, but has stability issues that affect the lifetime usage of the nanotree structure.


