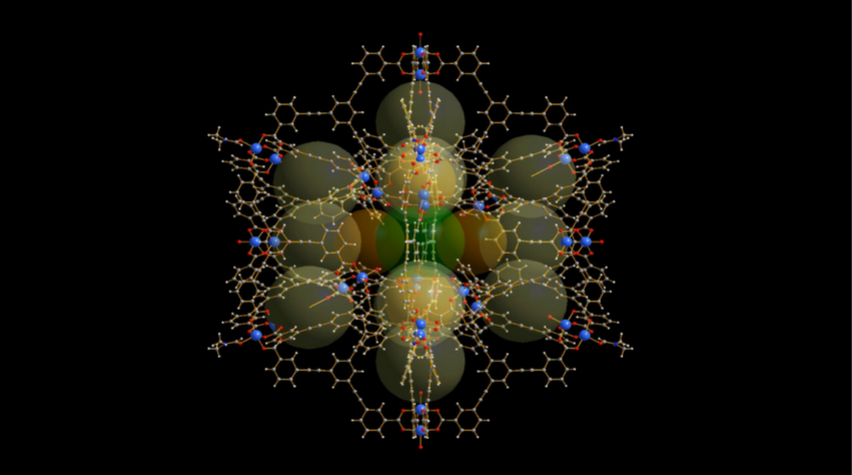
The structure of the newly developed "molecular cage," or MOP.
Scientists have developed a large metal-organic-organic polyhedra, or MOP, whose enormous intrinsic surface area and solubility show great potential for energy conversion, drug delivery, and sensors. Among the largest ever developed, this MOP is composed of 36 copper atoms and is made up of 96 individual components.
Internal sub-cages are key differentiating feature
Within the cage-like structure of the MOP, which is made of up a number of sub-cage structures, different molecules can be discretely contained. Because of this divided structure, materials can be loaded into the MOP to react only under specific conditions.
This is particularly useful for bio-sensing and drug-delivery applications, where a biological cue would be required to initiate a chemical reaction. For example, a drug could be encapsulated in one of these MOP and be released at the specific target site, where a specific biological molecule would trigger its release.
The researchers point out that the possibility of confining distinct chemical reactions within sub-cages also presents the potential to mimic biological enzymes.
Green energy is the ultimate goal
The researchers behind the MOP also hope to develop light-active porous, metal-organic materials for use in green energy. Their aim is to create a molecule that could simply use light to convert energy as plants do.
This breakthrough is the work of researchers from Trinity College Dublin and AMBER, the Science Foundation Ireland-funded materials science research center hosted in Trinity College Dublin. Their published findings can be found in the journal Nature.
Image: Professor Wolfgang Schmitt, Trinity College Dublin
Log in to post comments


