2022 Annual Meeting
(99a) Activation of Lattice and Adatom Oxygen By Highly Stable Ceria-Supported Cu Single Atoms
Authors
Gregory Collinge - Presenter, Pacific Northwest National Laboratory
Carlos G. Vargas, Washington State University
Mal-Soon Lee, Pacific Northwest National Laboratory
Ya-qiong Su, Eindhoven University of Technology
Xavier Isidro Pereira Hernández, Washington State University
Dong Jiang, Washington State University
Vassiliki-Alexandra Glezakou, Pacific Northwest National Laboratory
Emiel Hensen, Eindhoven University of Technology
Roger Rousseau, ORNL
Abhaya Datye, University of New Mexico
Yong Wang, Pacific Northwest National Laboratory
Dongmin Yun, Washington State University
Valery Muravev, Eindhoven University of Technology
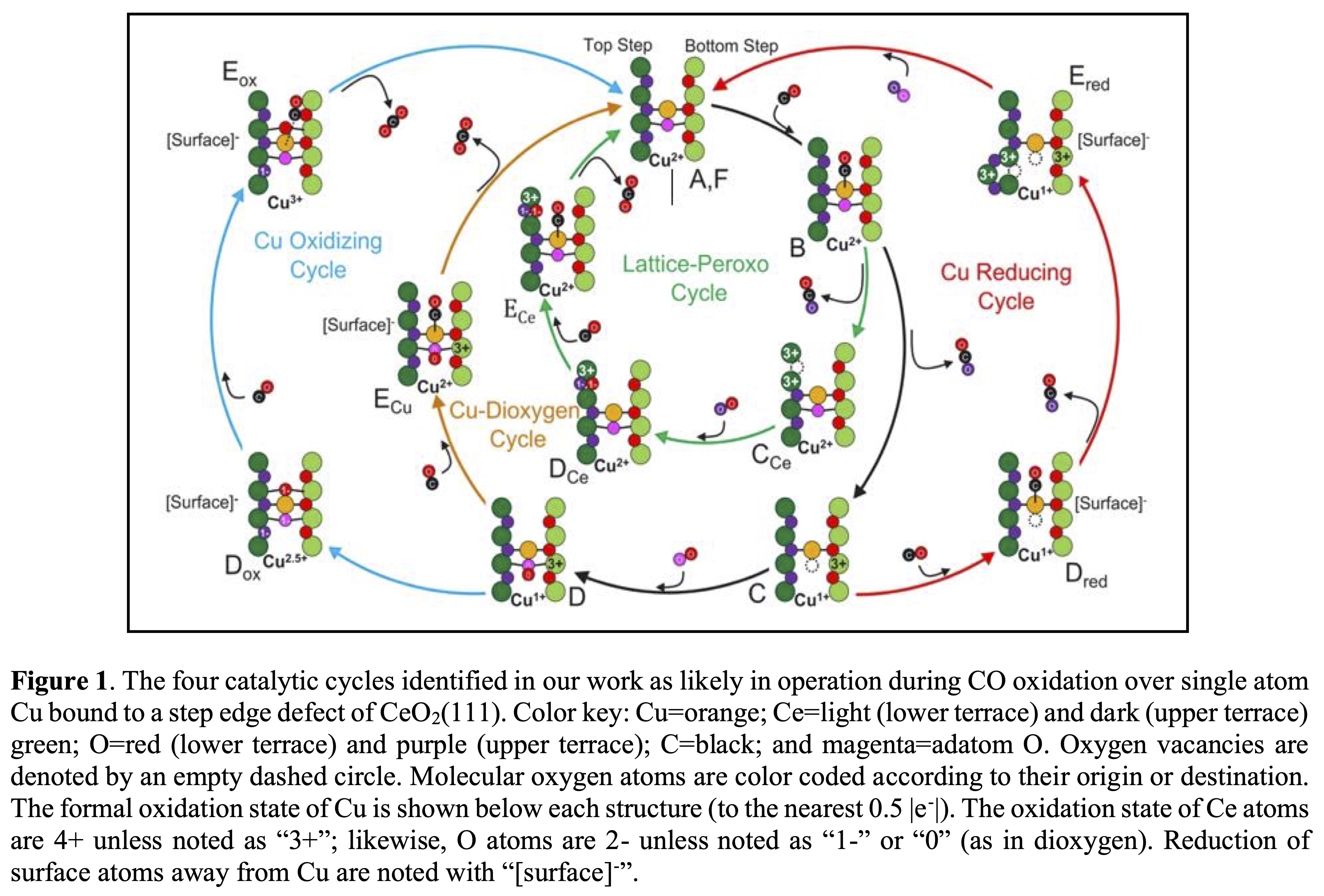
Requiring CO oxidation catalysts to be both active yet stable over long periods of time under variable reaction conditions, especially in the high and low temperatures typical to vehicle exhaust conditions, is a daunting challenge due to the almost mutual exclusivity of these constraints. Here, we demonstrate how thermally stable single atom Cu catalysts prepared by high temperature synthesis (atom trapping) on CeO2 are made to achieve this feat through a fully integrated experimental and computational approach. We combine kinetic measurements, CO temperature-programed reactions, XRD, XAS, STEM, XPS, and DRIFTS measurements with state-of-the-art DFT and activation barrier calculations along with calibrated charge density partitioning and formal charge analysis to probe this system and create a productive, prediction-focused feedback loop between experiment and theory. Our results indicate that single atom Cu, trapped in step defects, achieves its robust activity through facile charge transfer between active site and support (see the wide array and disparity of charge states computed and labeled in Figure 1). This provides the catalysts with an ability to activate either lattice (red and purple circles) or adatom (magenta circles) oxygen atoms, accessing additional reaction channels/catalytic cycles depending on the reaction conditions. Such adaptability allows dynamic response of this catalyst to variable reaction conditions that allows it to manifest at least one viable and active reaction pathwayâif not multiple. The inherent stability of the catalyst arises from the enhanced strength of Cu-O interactions, which are maximized during high temperature synthesis and exist even when Cu oxidation state varies, retarding sintering and deactivation. As we will show, one can circumvent the dilemma of designing catalysts that are simultaneously active and stable by matching the redox properties of single atom catalysts to those of the support to establish an environmental adaptability around the active sites.