2022 Annual Meeting
(656a) A New Computational-Experimental Screening Methodology Identifies More Effective Solvents for CO2 Capture
Authors
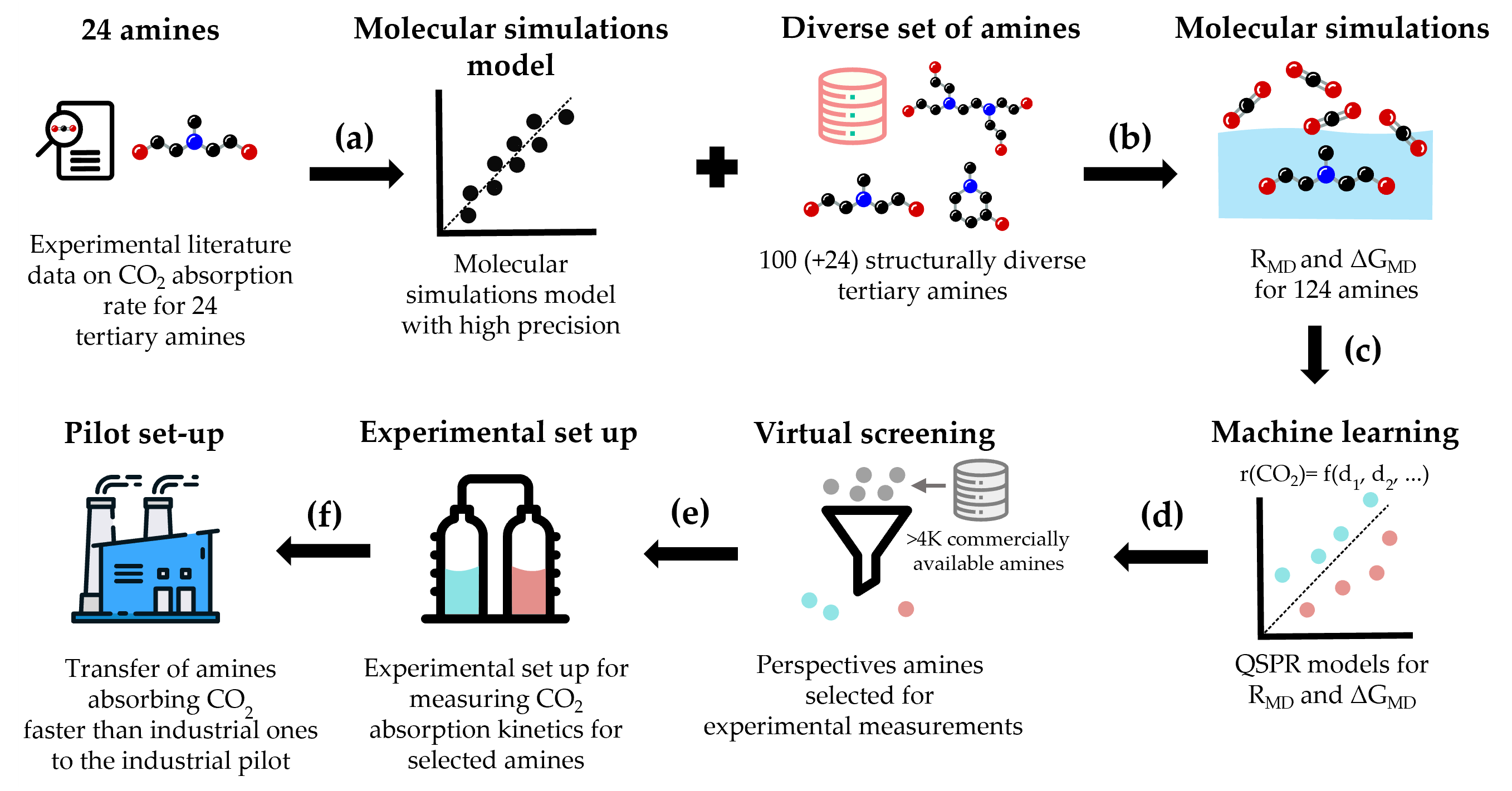
Figure 1 illustrates the workflow of the methodology. First, we have developed a computational approach based on molecular modeling to predict the CO2 absorption rates and energies in aqueous tertiary amines. The predictions are validated using experimental data. The key to the new model's excellent accuracy is to include the solvation effects in the computation of the free energy barrier for the reaction of CO2 absorption [1].
In a second step we have generated a diverse library of 100 tertiary amines which represent a large portion of chemotypes relevant for industrial amine-based chemical CO2 absorption. Using the molecular simulations-based model, we have calculated the CO2 absorption rates and energies for those 100 aqueous amine solvents. As a check, some points have been verified experimentally [2].
In a third step we have used the dataset of the CO2 absorption rates and energies for the 100 aqueous amines to build quantitative structure-property relationship models. These models allowed us to perform a large-scale virtual screening of commercially available amines and to identify the amines with optimal CO2 absorption rate and energy. For several amines the predictions have been verified experimentally. Subsequently, for the best performing amine, piperazine has been added. The approach and the results will be presented.
[1] Rozanska, X., Wimmer, E. & de Meyer, F. Quantitative Kinetic Model of CO2 Absorption in Aqueous Tertiary Amine Solvents. J. Chem. Inf. Model. 61, 1814â1824 (2021)
[2] Orlov, A., Valtz, A., Coquelet, C., Rozanska, X., Wimmer, E., de Meyer, F. Computational screening methodology identifies effective solvents for CO2 capture. Communications Chemistry 5, 37 (2022)