2022 Annual Meeting
(641a) Comparison of One Dimensional and Two-Dimensional Population Balance Model for Optimization of a Crystallization Process
Authors
Crystallization is a key unit operation in the isolation of many active pharmaceutical ingredients. The crystallization process commonly has a significant impact on the quality of drugs and the efficiency of downstream processes such as filtration, milling, centrifugation, drying, granulation, and tableting (Eren et al., 2019). One of the key challenges in industry is the integration of Quality-by-Design methods when designing unit operations and processes. This would help to provide potential ways to improve quality while reducing variability and is being actively encouraged by pharmaceutical regulatory agencies (Allison et al., 2015). The two-dimensional population balance model (2D PBM) allows for the modelling of two different growth kinetics, along perpendicular major & minor axes of a particle. This approach has several advantages for the modelling crystallization processes that generate non-spherical particles, such as needles and plates, commonly encountered for active pharmaceutical ingredients (API) (Rosenbaum et al., 2022). The 2D PBM approach is also able to account for morphological evolution during a crystallization process; something that cannot be successfully accomplished with a 1D model.
Results:
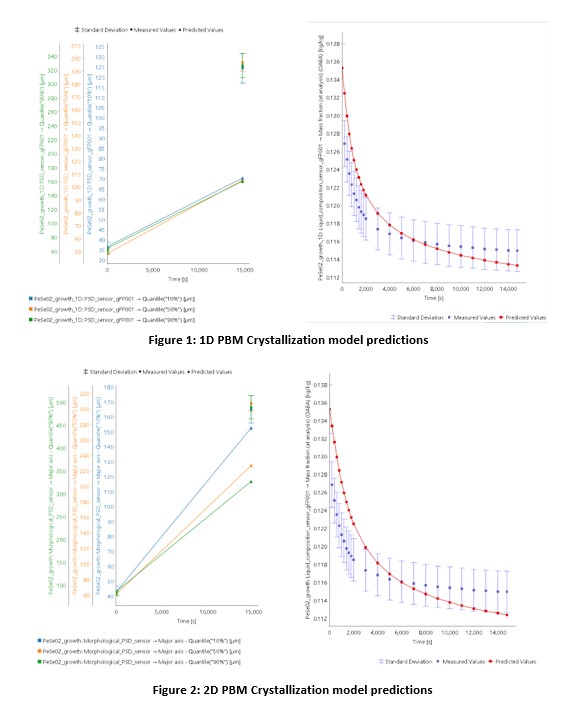
This work outlines a stepwise approach to configuring, validating and optimizing a 2D PBM model. As a first step, the experimental data gathered is used to configure and validate a 1D PBM model. The initial validated 1D model showed that while the desupersaturation behaviour is well predicted and can be optimized using a 1D approach, the particle size did not match the PSD quantiles observed experimentally as shown in Figure 1 (attached).
From these findings, it is evident that the particle shape changes during the crystallization process, which results in a change in particle shape, something that the 1D approach is not able to account for. Therefore, to investigate this further, a 2D PBM model is configured and validated using the estimated model parameters from the 1D approach as initial guesses. The results indicate that the 2D approach is better equipped to predict the particle shape evolution during the process, as shown in Figure 2 (attached)
These results were used to explore the design space and optimize the crystallization process with a view of identifying process parameters which may results in an improved product particle, namely lower aspect ratio (major axis/minor axis).
References
Allison, G., Cain, Y. T., Cooney, C., Garcia, T., Bizjak, T. G., Holte, O., . . . Zezza, D. (2015). Regulatory and quality considerations for continuous manufacturing. May 20-21, 2014 Continuous Manufacturing Symposium. J Pharm Sci, 104(3), 803-812.
Eren, A., Szilagyi, B., Quon, J., Furuta, M., Papageorgiou, C., Nagy, Z., . . . Ozkan, L. (2019). Development of a Model-Based Quality-by-Control Framework for Crystallization Design. 29th European Symposium on Computer Aided Process Engineering, Pt a, 46, 319-324. doi:10.1016/B978-0-12-818634-3.50054-0
Rosenbaum, T., Mbachu, V., Mitchell, N. A., Gamble, J. F., Cho, P., & Engstrom, J. D. (2022). Comparison of One-Dimensional and Two-Dimensional Population Balance Models for Optimization of a Crystallization Process for a Needle-Shaped Active Pharmaceutical Ingredient. Organic Process Research & Development. doi:10.1021/acs.oprd.1c00344