2022 Annual Meeting
(631d) Comparison between Two Different 3D Printed Tumor-on-Chip Devices As Anti-Cancer Testing Platforms
Authors
Here we describe the development and comparison of two micro/mini fluidic platforms aimed to contribute to the advance of tumor-on-chip research and based on 3D-printed devices.
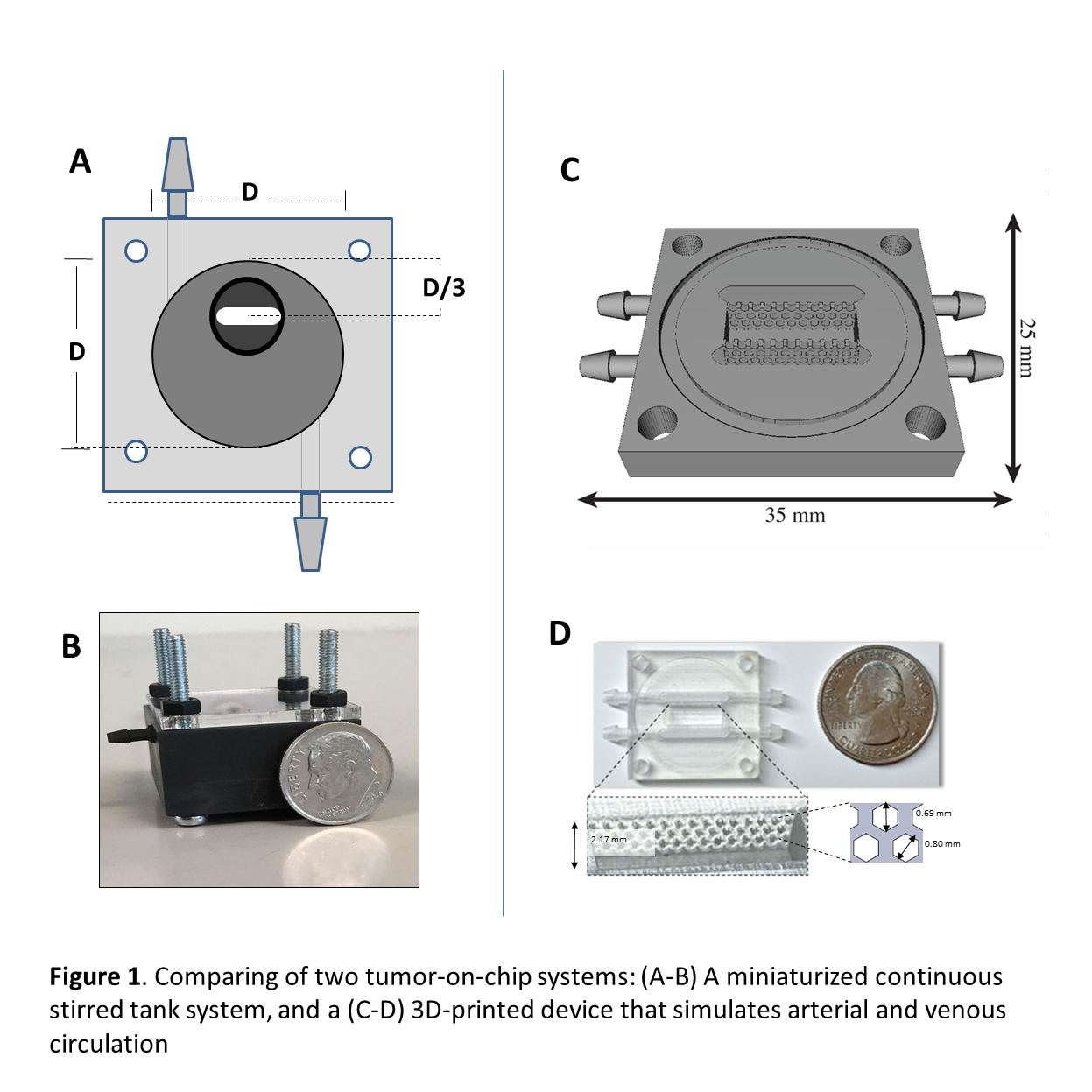
First, we describe a simple and robust method for the fabrication, maturation, and extended culture of large breast cancer (MCF7 and MCF7/fibroblasts) spheroids (~800 µm in diameter) in a 3D-printed mini continuous stirred tank reactor (mini-CSTR) (Figure 1 A-B). In brief, MCF7 cell suspensions (5Ã104 cells) were incubated in batch culture for three days in ultra-low-adherence (ULA) plates to form discoid cell aggregates (700â800 µm in diameter). These microtissues were then transferred into the mini-CSTR and continuously fed with culture media for an extended time (~30 days). The spheroids progressively increased in size during the first 20 days of perfusion culture to reach a steady diameter. At low residence times (~22 h) and glucose concentrations of 1.1 g L-1, the spheroids reached a diameter of 700-800 µm. We characterized the spheroid morphology and architecture (i.e., shape, size, and cell viability at different radial zones within the spheroid), and the evolution of expression of relevant tumor-related genes (i.e., ER, VEGF, Ki67, Bcl2, LDHA, and HIF-1α) in spheroids cultured for 30 days.
This mini-CSTR culture strategy enables the simple and reproducible fabrication of relatively large spheroids and offers great potential for studying the effects of diverse effectors on tumor progression. Moreover, we used the mini-CSTR system to evaluate the effect of the continuous perfusion of three anti-cancer drugs (i.e., docetaxel, paclitaxel, and doxorubicin) on large MCF7-derived spheroids. Our results show that the use of this agitated system as a tumor-on-chip platform may be a useful and practical tool for testing the efficacy and safety of novel anti-cancer drugs and may enable personalized medicine applications.
In addition, we introduce 3D-printed microfluidic system (Figure 1 c-D) that can be generically used to culture tumor-tissues under well-controlled environments. The system is composed by three compartments. The left and right compartments have one inlet and one outlet each, providing means to continuously feed two liquid streams to the system. The central compartment is designed to host a cell-laden hydrogel where a microtissue can be confined and cultured. A transparent lid and bottom can be adapted to enable visual inspection under a microscope.
We conducted fluorescent and saline tracer experiments to characterize the hydraulic performance of the system. In addition, we cultured MCF7-spheroids embedded in a cell-laden hydrogel construct (placed in the central chamber), to illustrate the use of this system to sustain long term micro-tissue culture experiments. We also present experimental results that illustrate the flexibility and robustness of use of this 3D-printed device for tumor-on-chip experiments that including pharmacological testing of individual and combined anti-cancer compounds (i.e., docetaxel and docetaxel/paclitaxel).
These âopen-sourceâ organ-on-chip systems are intended to be a general-purpose resource to facilitate/democratize the development of tumor-on-chip applications.