2022 Annual Meeting
(61d) Correlating Protein Motor Structure with Function Via Novel Biophysical Approaches
Author
Pushkar Lele - Presenter, Texas A&M Engineering Experiment Station
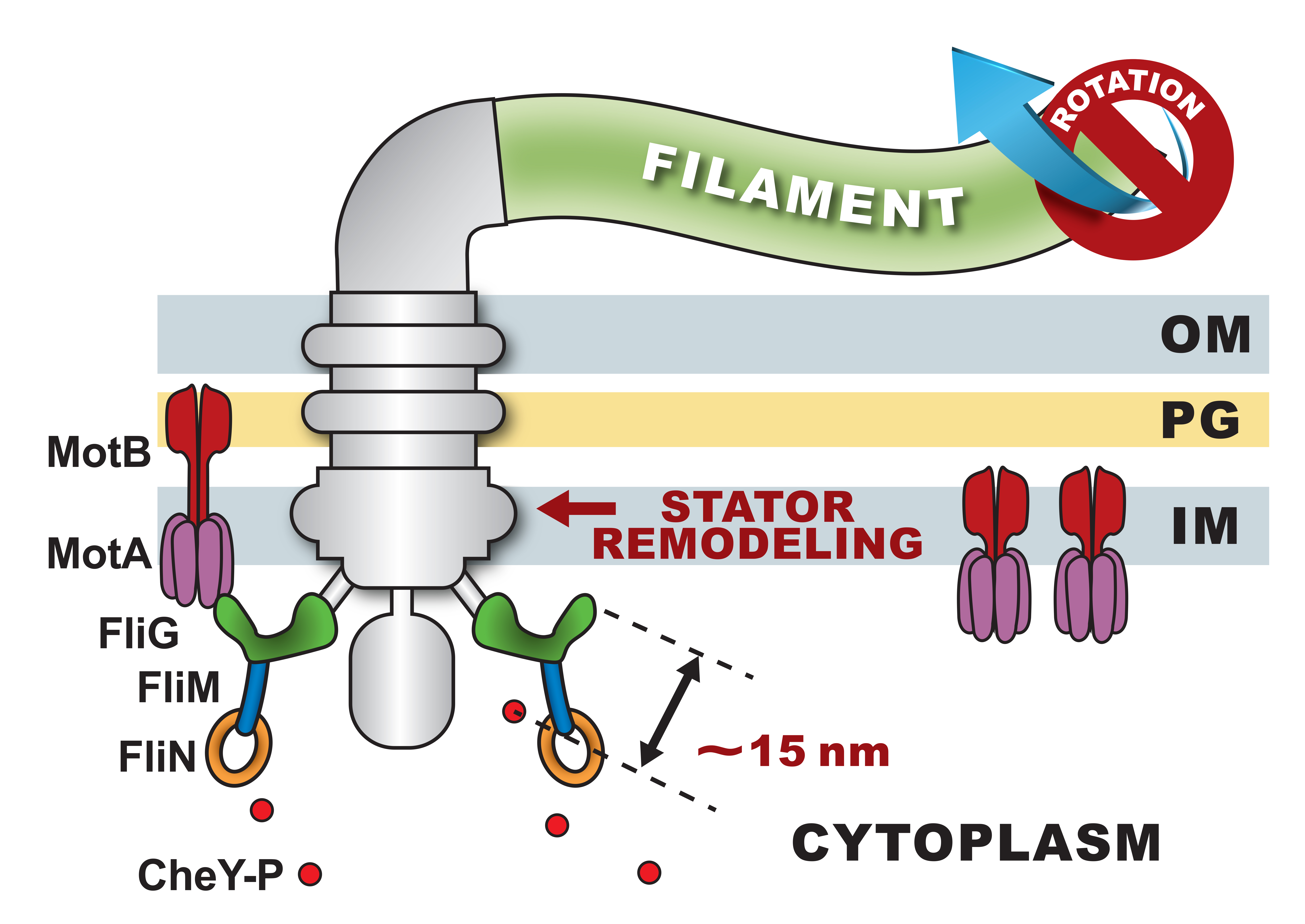
The revolution in single-particle cryo-EM techniques has provided unparalleled resolution in visualizing membrane proteins without crystallization. In bacteria, these methods have been successfully extended to study a variety of molecular motors. In particular, several high-profile publications[1,2,3] have revealed astonishing details that appear to suggest conserved symmetries in the structures of motors likely involved in cell division and outer membrane maintenance (TolQ-TolR), those that mediate transport of nutrients (ExbB-ExbD), and those involved in flagellar motility (MotA-MotB) and gliding motility (GldL5M2). On the basis of structural similarities, the prevailing view is that these motors are likely rotary in nature. This hypothesis hinges on MotA-MotB: a MotA pentamer assembles around a MotB dimer forming ion channels. The flux of protons through these channels is believed to power the rotation of the pentamer around the dimer to enable motility. I will discuss innovations in biophysical approaches that are enabling this hypothesis to be tested. I will elaborate on how bacterial proprioception[4] is likely to be coupled with MotA-MotB function. I will conclude with the broader implications of successfully explaining MotA-MotB operation for our understanding of bacterial physiology.
[1] Santiveri et al, Cell, 2020; [2] Deme et al, Nature Microbiology, 2020; [3] Chang et al, Nature Structural & Molecular Biology, 2020; [4] Antani et al, Nature Communications, 2021.