2022 Annual Meeting
(618d) Tuning Selectivity of CO2 Methanation over MgO-Ni/SiO2
Authors
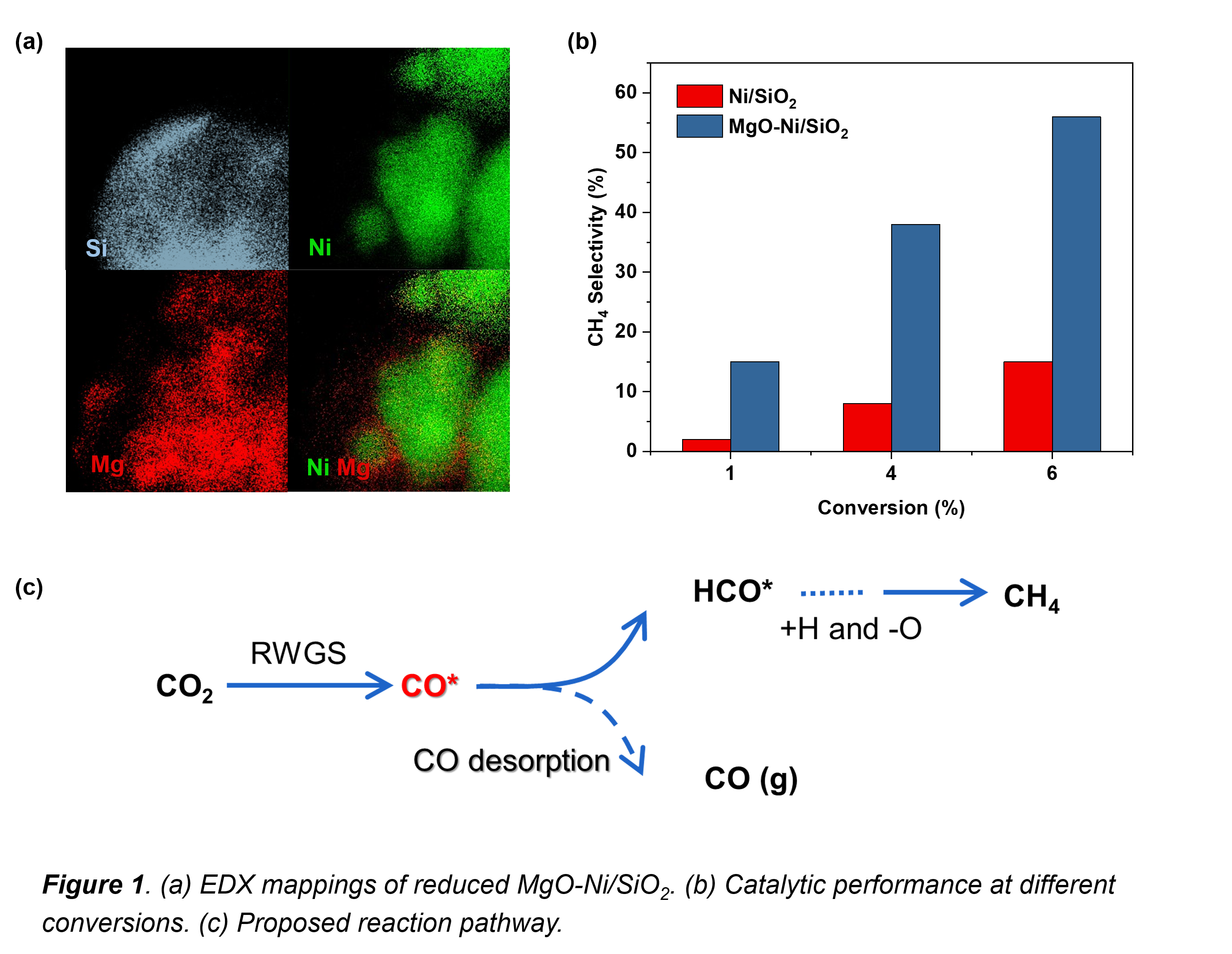
MgO-Ni/SiO2 catalysts with varying MgO were prepared by sequential incipient-wetness impregnation. For MgO loadings from 0 to 3 wt.%, the Ni metallic surface area decreased by half, indicating MgO covered part of the metallic Ni, which was confirmed by HRSTEM-EDX (Figure 1(a)). In situ QXAS during H2-TPR showed that NiO in the as-prepared samples could be completely reduced into metallic Ni.
Catalytic performance tests showed that MgO addition dramatically increased the CH4 selectivity at the same CO2 conversion (Figure 1(b)). As MgO itself is hardly active, the selectivity increase was ascribed to the interaction of Ni and MgO. The formation of CH4 from CO2 might follow two pathways, direct or indirect with CO* as the key intermediate. Kinetic and in situ DRIFTS tests point towards the indirect route. Moreover, transient in situ DRIFTS proved that on the MgO-added samples the transformation CO â HCO* â CH4 occurs faster.
This work successfully constructed a methanation catalyst, where MgO partially covered metallic Ni. The mechanism of the CO2 methanation process over MgO-Ni/SiO2 was detailed (Figure 1(c)), as well as the significance of the MgO/Ni interface, which is critical to develop new, highly active and selective catalysts for CO2 utilization.