2022 Annual Meeting
(605e) Digital Process Replica of RNA Manufacturing for Enabling Process Optimization, Technology Transfer and Techno-Economic Assessment
Authors
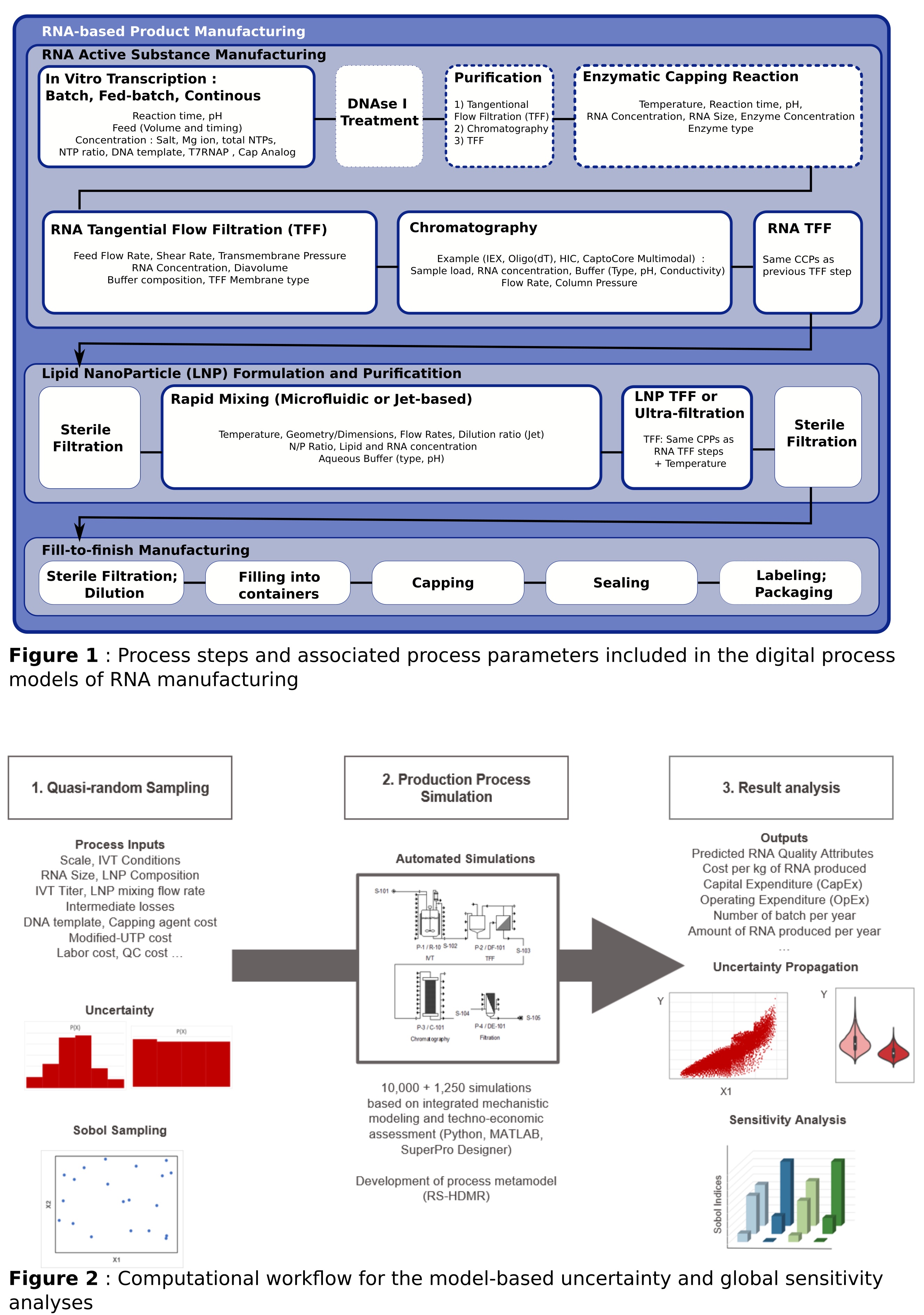
Our approach starts with an extensive review of relevant, large-scale, cGMP RNA manufacturing processes, encompassing current Covid-19 vaccines production settings and potential innovations. This enables the identification of crucial process steps, critical process parameters, and relevant manufacturing options to be simulated (See Figure 1). In this study, particular emphasis is placed on the In Vitro Transcription (IvT) modes of operations and RNA 5â-capping options. Then, process models are built for each unit procedure to integrate IvT kinetic modelling with downstream and formulation simulations. This holistic modelling approach allows the prediction of the main key performance indicators (KPIs) and critical quality attributes (CQAs) under different scenarios. This includes RNA yield, recovery, process time, production cost, RNA degradation, and the formation and removal of product-related impurities throughout the manufacturing process. This model-based assessment is however limited by the lack of transparency, as well as by inherent variabilities and unknowns surrounding process design, equipment, and location. A sensitivity analysis is thus performed to embrace these uncertainties. A variance-based global sensitivity analysis has been selected to perform 10,000 quasi-random sampling based on low-discrepancy Sobol sequences. These simulations are used to explore the input-output uncertainty propagation and compare the different manufacturing options in a risk-based manner. Random Sampling-High dimensional Model Representation (RS-HDMR) metamodeling is also implemented to obtain an accurate estimation of the sensitivity indices, indicating the degree to which these variables influence process performance and product quality (see Figure 2).
Results
First, process simulations confirm the high production capacity of the RNA technology. The equivalent of hundreds of millions of Covid-19 vaccines doses could be produced each year by a single production line. However, the sensitivity analysis reveals significant discrepancies. The LNP formulation step is identified as the production bottleneck in most cases. Thus, the choice of mixing method, device parallelization and numbering is critical to ensure optimal annual productivity, and is highly dependent on the IvT mode of operation and expected yield. Secondly, in sharp contrast with other biopharmaceutical productions, the consumptions of IvT raw materials and capping agent are the main cost drivers. For this reason, the fed-batch approach is economically attractive, especially in combination with co-transcriptional capping. The trade-off, however, is an additional production of RNA-related impurities, further increasing downstream burden to obtain high quality products. Similarly, the application of high-temperature conditions can make the enzymatic capping approach cost competitive. Ultimately, the transition from batch to continuous operations is highly attractive in terms of process transferability and product quality. The optimization of process occupancy would enable the development of a smaller and cheaper facilities. IvT reactor scale can be at least divided by 10, further reducing the footprints of all other downstream units. Process streamlining can finally lead to raw materials recycling, making the vaccine supply chain more robust and the process more cost-efficient
Implications and future perspective
This work represents the first end-to-end, integrated modelling of RNA manufacturing processes encompassing different manufacturing options. This process digitization is particularly useful in such nascent platform technology. It provides a reliable tool for assessing the requirements for setting up a new facility and can help would-be manufacturers establish a suited manufacturing strategy. This analysis also points out where process intensification and development are most needed in order to optimize manufacturing capabilities, process transferability, production costs and product quality. We further showcase that continuous manufacturing is technically feasible and economically competitive. This switch to continuous operations could pave the way towards the deployment of a transferable, distributed, and affordable RNA platform technology. In another perspective, the identified design space and modelling capabilities could be at the basis of future model-based design of experiments and process design. Finally, these models could be expanded and complexified in the near future. The integration of process analytical technology (PAT) tools would notably increase our prediction capability and support the use of model-based process control.