2022 Annual Meeting
(566c) N-Glycanyzer: An Integrated on-Line Process Analytical Toolkit for Enabling Continuous Biologic Manufacturing
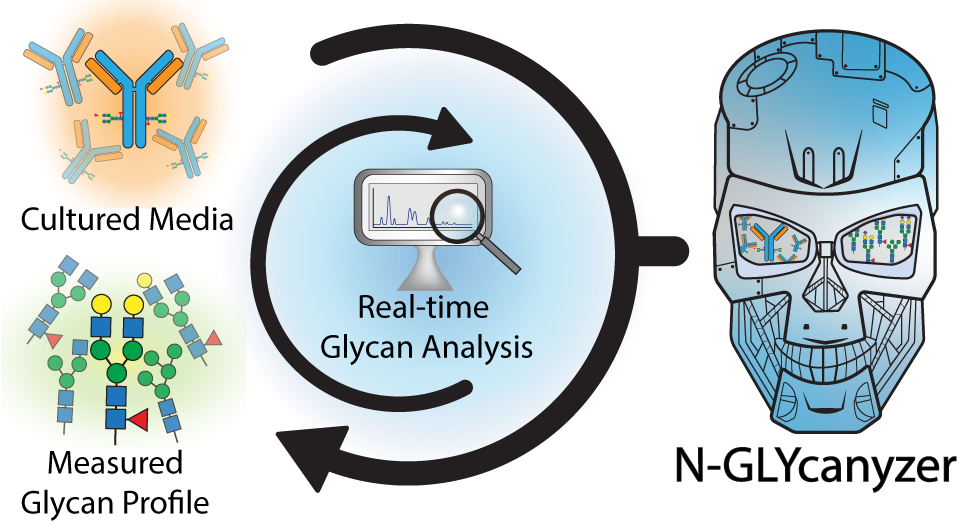
The biopharmaceutical industry is transitioning towards the adoption of continuous biomanufacturing practices that are often more flexible and efficient than traditional batch processes. Federal regulatory agencies like the FDA are further urging use of advanced Process Analytical Technology (PAT) to analyze the design space to increase process knowledge and enable high quality biologics production. Post-translational modification of proteins, such as N-linked glycosylation, are often critical quality attributes that affect biologics safety and efficacy, requiring close monitoring during manufacturing. Here we have developed the N-Glycanyzer as an integrated PAT tool that combines on-line sampling and sample preparation with a mobile HPLC unit allowing for automated and near-real time measurement of monoclonal antibody (mAb) glycosylation (1). This toolkit can be used to understand the temporal changes of glycosylation during fed-batch and perfusion based mAb manufacturing allowing for tighter control of the glycan profile, leading to less variability during upstream bioprocessing. We will highlight the key unit operations and optimization techniques used to build the N-GLYcanyzer PAT tool and provide an example of its use for monitoring a fed-batch upstream process using mammalian cells for producing a commercially relevant Trastuzumab biosimilar.
1. Gyorgypal, A. & Chundawat, S. P. S., "Integrated Process Analytical Platform for Automated Monitoring of Monoclonal Antibody N-Linked Glycosylation," Analytical Chemistry.analchem.1c05396 (2022). DOI: 10.1021/acs.analchem.1c05396. Weblink: https://pubs.acs.org/doi/10.1021/acs.analchem.1c05396