Ionic liquids (ILs) have recently been studied for the design of more efficient and sustainable industrial processes such as the recycling of rare earth elements (REEs).
1 Separation processes have been proposed that leverage the differences of thermodynamic and transport properties between REE and IL complexes under external electric fields (EEF).
2,3 However, studies on the effect of EEF on ILs have been limited to evaluating transport properties and structure.
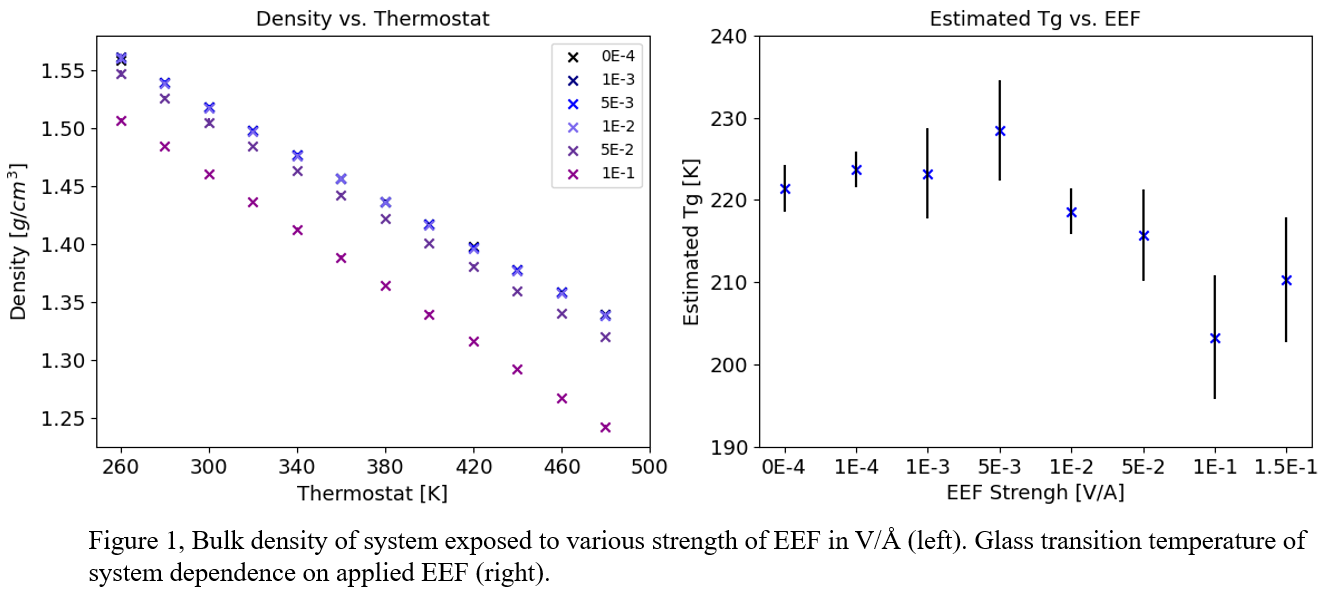
4,5 To understand the effects of the EEF on properties relevant to REE unit design such as bulk properties and phase behavior, we present the use of molecular dynamics (MD) simulations of the ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C2C1im][NTf2]) in the presence of EEFs of varying strength to elucidate this emergent behavior. Simulations show a drastic drop in glass transition temperature and overall decrease in density once exposed to a high EEF shown in figure 1. Also, MD simulations show a significant increase in self-diffusion coefficients once a critical EEF strength is applied. Specifically, movement in the direction of the applied EEF is facilitated by the force experienced by the charged ions. This facilitates diffusion in the direction of the applied EEF for the [C2C1im] cation and the opposite direction for the [NTf2] anion. In addition, radial distribution functions indicate a decrease in ordering in the liquid structure with increasing electric field strength. These effects indicate that the EEF fluidizes the IL, allowing for the system to sample more configurations in the liquid phase.
References
(1) Kaiying Wang; Hertanto Adidharma; Maciej Radosz; Pingyu Wan; Xin Xu; K. Russell, C.; Hanjing Tian; Maohong Fan; Jiang Yu. Recovery of Rare Earth Elements with Ionic Liquids. Green Chemistry 2017, 19 (19), 4469â4493.
(2) Hussain, A.; AlAjmi, M. F.; Hussain, I.; Ali, I. Future of Ionic Liquids for Chiral Separations in High-Performance Liquid Chromatography and Capillary Electrophoresis. 2018, 49 (4), 289â305.
(3) Gangu, Satya Aravind. Towards In situ extraction of fine chemicals and biorenewable fuels from fermentation broths using Ionic liquids and the Intensification of contacting by the application of Electric Fields. Diss. University of Kansas, 2013.
(4) Toda, S.; Clark, R.; Welton, T.; Shigeto, S. Observation of the Pockels Effect in Ionic Liquids and Insights into the Length Scale of Potential-Induced Ordering. Langmuir 2021, 37 (17), 5193â5201.
(5) English, N. J.; Mooney, D. A.; OâBrien, S. Ionic Liquids in External Electric and Electromagnetic Fields: A Molecular Dynamics Study. Molecular Physics 2011, 109 (4), 625â638.