2022 Annual Meeting
(532ad) Glucose Oxidation to Aldonic and Aldaric Acids Using Molecular Oxygen
Authors
Glucaric acid is an aldaric acid, which was already discussed in the late 1800s. The first person to document it was Kiliani, who prepared it by oxidizing D-glucose with nitric acid. The utility of glucaric acid is an excellent motivator for the development of an industrially applicable environmentally friendly production process.
In our work, we were trying to determine the origin of selectivity in glucose oxidation using molecular oxygen. We synthesized a monometallic catalyst (Au) and bimetallic catalysts, where we alloyed the gold with other metals (AuPt, AuCu, and AuRe). The support in all cases was zirconium dioxide. The former catalyst contained 7 wt.% of Au, while in the latter materials, we replaced 3.5 wt.% of Au with the appropriate molar amount of the alloying metal.
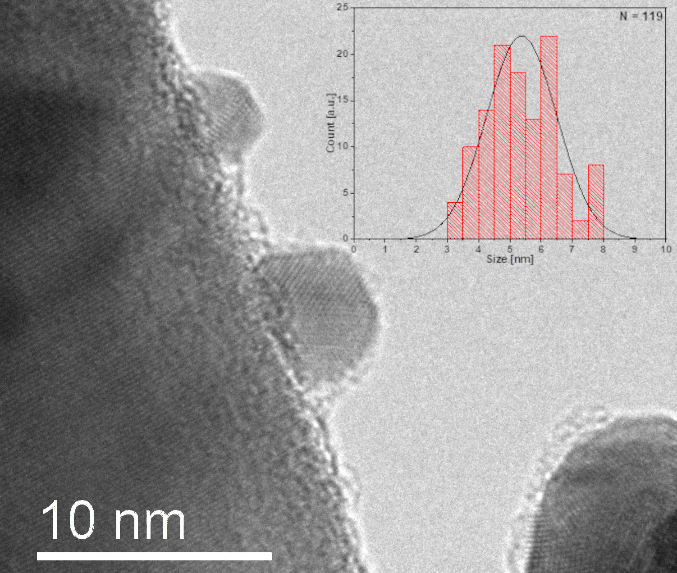
The catalysts were first analyzed by XRD, which revealed that only minimal diffraction peaks for gold are observed. We confirmed the bimetallic nature of the active metal particles by the selected area diffraction analysis, which confirmed the formation of alloys (Figure 1).
Glucose oxidation was performed in stirred batch reactors Parr autoclave. We conducted the reactions from 60 to 120 °C with a partial pressure of oxygen of 16 or 30 barg. Glucaric acid was detected only when Cu or Pt were present in the material. With the data, we gathered, we composed a tentative reaction mechanism. We found, that at higher partial pressures of oxygen, over-oxidation of glucaric or gluconic acids can occur. Additionally, we constructed a microkinetic model for a three-phase system. With it, we calculated reaction rate constants and activation energies for each reaction step.