2022 Annual Meeting
(459d) Scale-Down of Viral Inactivation Step: Principles and Practical Challenges
Author
Zbynek Kalal - Presenter, Merck & Co.
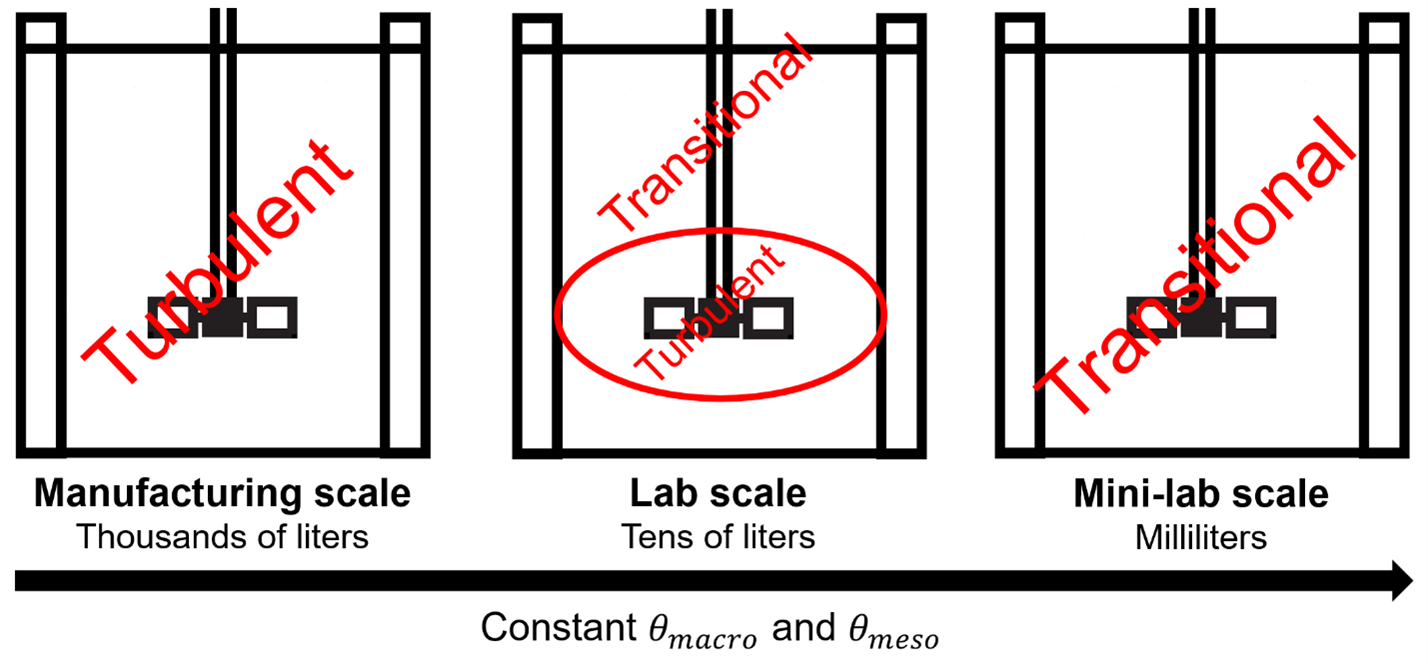
The low-pH viral inactivation (VI) step is a process used to inactivate potential enveloped viruses in biological drugs manufacturing. The step typically involves a low-pH titration of a chromatography pool using an acid titrant. Defining agitation conditions for the process is important to avoid pH gradients in the protein pool, which could cause higher levels of impurity formation. The typical approach to optimize a low-pH VI step is through utilization of a small-scale model. Developing such a model is complicated by the fact that the flow regime in the small-scale tank may not be representative of the manufacturing-scale system. In this presentation we identify the critical process parameter that defines the process outcome, outline the principles of scaling down the VI process in order to ensure a representative mixing profile in the laboratory system, and highlight the challenge of scaling down to tank sizes that are manageable from a protein utilization perspective.