2022 Annual Meeting
(453c) Pt-Based Monolithic Catalysts for Oxidative Coupling of Methane: Effect of Catalyst Formulation and Operation Parameters
Authors
Over the decades, significant research has been conducted on methane as possible platform chemical for various reactions [1,2]. Amongst these, the oxidative coupling of methane (OCM) has been subject to many studies elucidating the influence of the active material (e.g. MnNa2WO4, Pt, and other metals) on the formation of C2 species [3-5]. Recently, Chawla et al. [4] investigated a monolithic Pt/Al2O3 catalyst for acetylene formation at high temperatures and used computational methods to understand the complex OCM mechanism over Pt, which can ultimately contribute to improving the catalystâs activity and product selectivity. Furthermore, studies on 3D printed monoliths by Bogdan et al. [5] suggest a strong correlation between catalyst geometry and C2 yields.
In our present study we investigate the influence of the monolithic structure together with the composition of the washcoat to reach higher acetylene selectivities. Changing the monolith length and the monolithic channel diameter as well as the support material and the washcoat thickness showed pathways to achieve higher acetylene selectivities.
Materials and Methods
The Pt catalysts were prepared by incipient wetness impregnation of the calcined metal oxide support materials Al2O3 and ZrO2. The received powder was coated onto a cordierite honeycomb monolith and fixed in a quartz glass tubular reactor. A heat shield in front of the catalyst ensured efficient heat transfer. The reactor was placed in a furnace and fed with a gas mixture consisting of methane, oxygen, and nitrogen to ignite the autothermal reaction at 500 °C. The exhaust gas was monitored by means of FTIR. Optimal reaction parameters were found by varying the N2 dilution between 85% and 30% and CH4:O2 ratios of 1.1 to 2.4 at a GHSV range of 68,000 h-1 to 1,200,000 h-1. In addition, the effect of different monolith cell densities (300, 400 and 600 cpsi) and varying washcoat composition was determined.
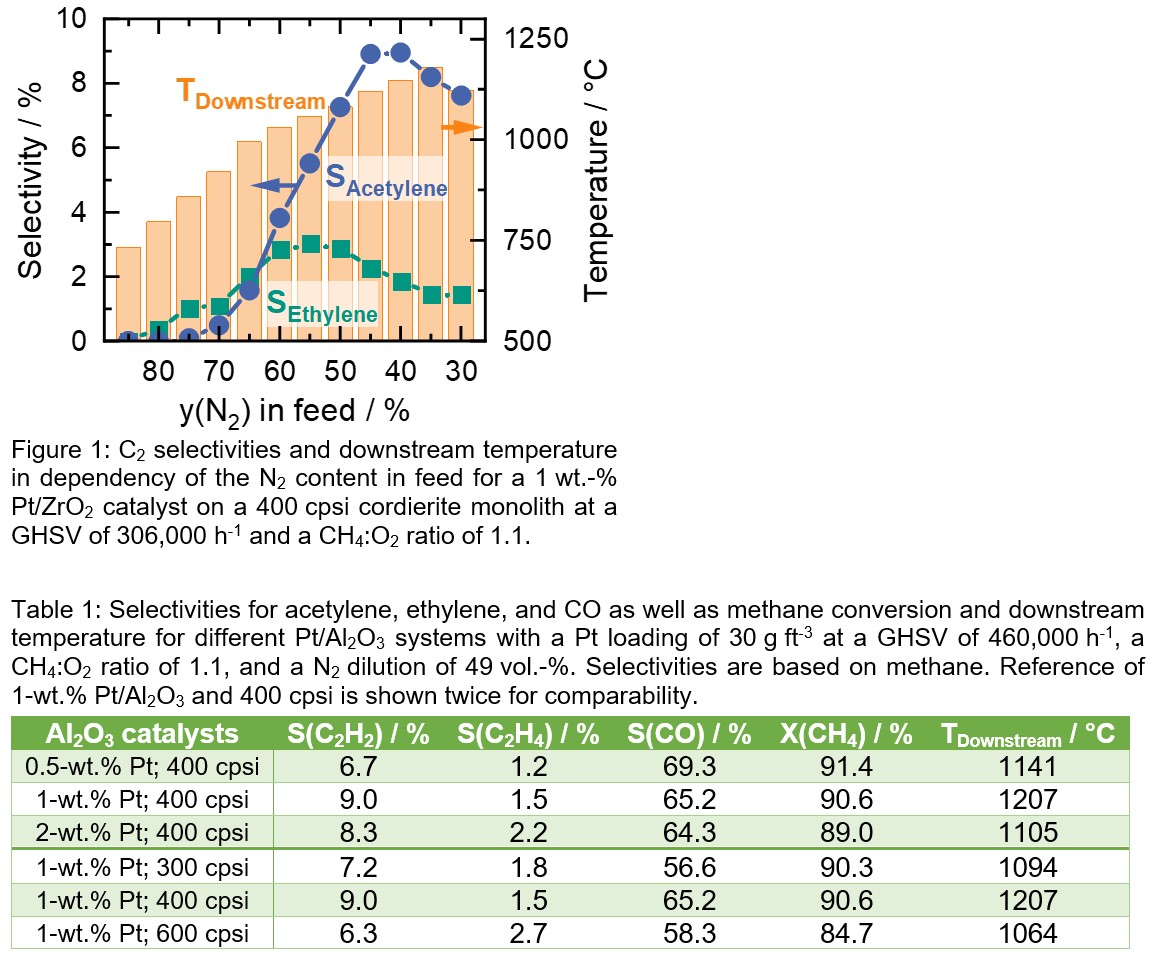
Results and Discussion
Figure 1 shows the influence of the N2 dilution on the OCM over a Pt/ZrO2 catalyst. The data suggest a strong connection between the N2 content in the feed and the downstream temperature as well as the C2 selectivities. With rising share of reactive gases (CH4 and O2), higher temperatures are achieved that lead to a higher C2 species formation. However, a maximum at 55 vol.-% and 40 vol.-% of N2 for ethylene and acetylene selectivity, respectively, is visible, whereas the highest temperature (~1180 °C) is found at 35 vol-%, suggesting temperature optimum for the gas phase reactions. Table 1 shows the influence of washcoat thickness over Al2O3 supported catalysts. As all listed catalysts exhibit a Pt loading of approx. 30 g ftâ3 and approx. identical particle size, higher Pt content in the used powder leads to a thinner washcoat, whereas the washcoat is approx. half the size for doubled Pt content (washcoat thickness: 0.5 wt.â% > 1 wt.â% > 2 wt.â%). The best performance was observed for the 1 wt.-% Pt/Al2O3 catalyst; the 2 wt.-% sample leads to a comparable total C2 selectivity but less acetylene in the product stream. Using a 0.5 wt.â% Pt/Al2O3 coating, exhibiting the thickest washcoat of the investigated catalysts, leads to more (partial) oxidation of methane and less C2 formation. Additionally, Table 1 shows the effect of different monolith geometries, proving 400 cpsi monoliths as most beneficial for OCM over Pt. By using smaller or bigger channel diameters ethylene formation seems promoted, but the overall desired C2 product formation is lowered. Looking at all cases depicted in Table 1, the influence of the reaction temperature (implied by TDownstream, measured 5 mm behind the catalyst) becomes visible. Although the highest temperature leads to the highest acetylene selectivity (1 wt.-% Pt; 400 cpsi), it does not seem to be the only important parameter. When comparing 0.5 wt.-% Pt; 400 cpsi with 1 wt.-%; 300 cpsi, a higher temperature with higher CO selectivity is observed for the first sample versus a lower temperature with a higher C2 selectivity for the latter one, respectively. This leads to the conclusion, that the monolithâs structure, including the washcoatâs composition, has a crucial impact on the product formation of the Pt-OCM.
Taking all the investigated parameters into consideration the 1 wtâ% Pt/ZrO2 catalyst on 400 cpsi exhibited the best results with 11.2% C2 selectivity and 89.1% CH4 conversion at a space velocity of 306,000 h-1 and a CH4:O2 ratio of 1.1.
References
[1] R. Franz, E.A. Uslamin, E.A. Pidko, Mendeleev Commun., 2021, 31, 584-592.
[2] O. Deutschmann, L.D. Schmidt., AIChE J., 1998, 44, 2465.
[3] G.E. Keller, M.M. Bhasin, J. Catal., 1982, 73, 9-19.
[4] J. Chawla, S. Schardt, S. Angeli, P. Lott, S. Tischer, L. Maier, O. Deutschmann, Catalysts, 2022, 12 (2), 189.
[5] E. Bogdan, B. Michorczyk, A. RokiciÅska, M. Basta, M. Myradova, P. KuÅtrowski, P. Michorczyk, Appl. Surf. Sci., 2021, 553, 149554.