2022 Annual Meeting
(42c) Fundamental Study of the Pretreatment Effect on the NiOx/CeO2 catalyst for NO+CO Reaction: Structure-Activity Relationship
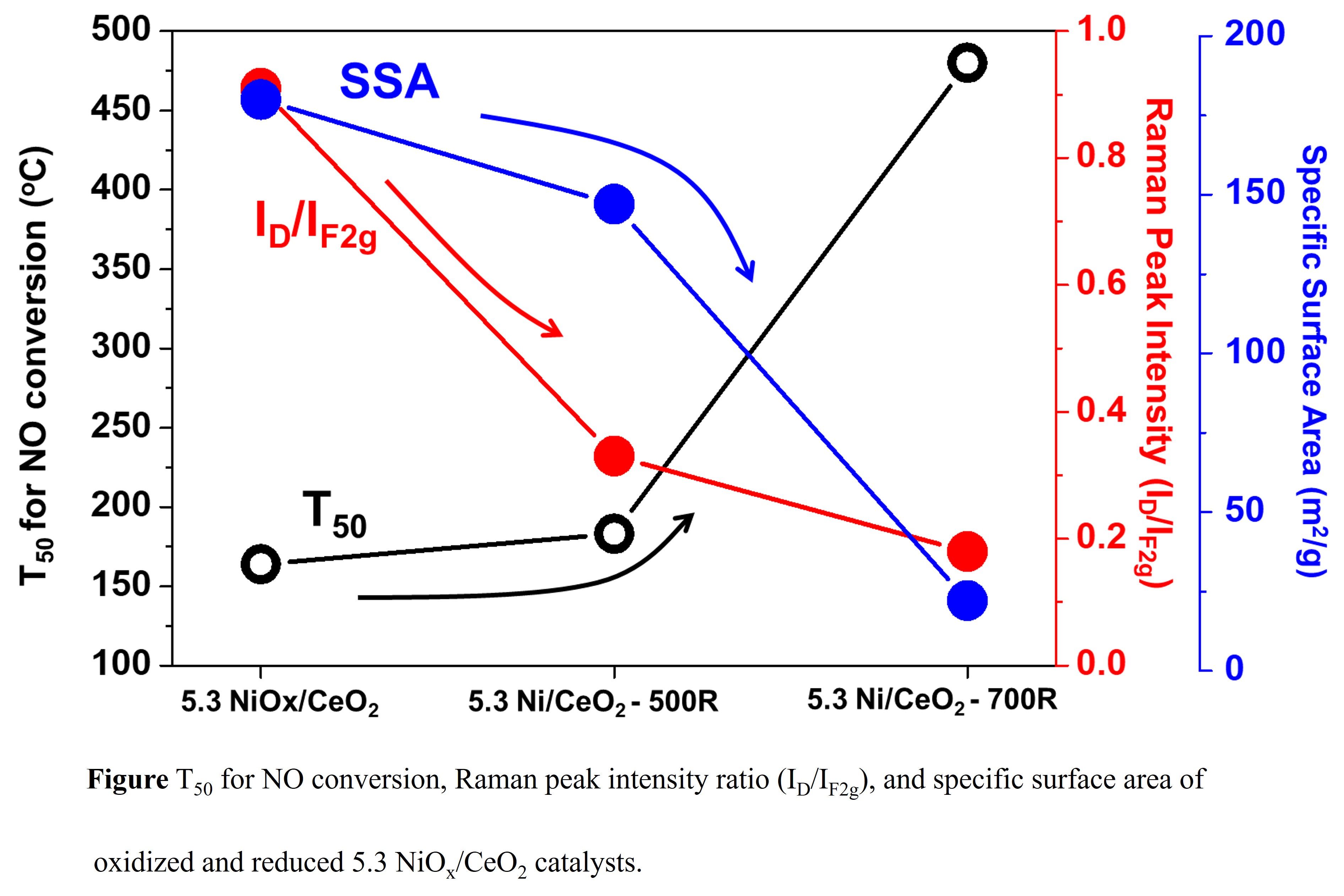
Nitrogen oxides (NOx) and carbon monoxide (CO) are considered as poisonous and severe atmospheric pollutants, which were mainly generated from stationery and automobile. NO reduction by CO reaction (2NO + 2CO â N2 + CO2) have attracted more attention because both pollutants generated from automobile exhaust, and it removes two pollutants (NO and CO) simultaneously. NiOx/CeO2 catalysts showed promising results in various NO reduction reactions, such as the selective catalytic reduction of NO by NH3 (SCR-NH3) and NO reduction by CO or hydrocarbon reaction. In this study, we investigate the effect of oxidation and reduction treatment on the NO reduction by CO reaction of pretreated NiOx/CeO2 catalysts. A series of oxidized and reduced CeO2 supports with different temperature (400oC for oxidation, 500 and 700oC for reduction) were used as the support materials, followed by the NiOx/CeO2 catalysts were synthesized by an incipient wetness impregnation method (IWI) with a fixed surface density of 5.3 Ni/nm2 and treated under specific conditions (oxidation/reduction), same as support treatment. The prepared catalysts were characterized via BET, Raman, and XRD to understand physiochemical properties. The results showed that as the reduction temperature increased, physiochemical properties of NiOx/CeO2 catalysts were changed: decreased specific surface area (SSA), decreased oxygen vacancy/defect site, decreased oxidation states of Ni, and larger crystallite size. The oxidized NiOx/CeO2 catalyst performed the best catalytic activity, indicating that the physiochemical properties of the catalysts (oxygen vacancy/defect site, SSA, crystallite size, and oxidation states) were key factors to enhance the catalytic activity, rather than surface density of the catalysts.