2022 Annual Meeting
(377f) Characterizing Rh Single-Atom Catalysts on ?-Al2O3 Using Density Functional Theory and CO Probe Molecule IR Spectroscopy
Authors
Alexander Hoffman - Presenter, University of Florida
Nicholas Gadinas, University of California - Santa Barbara
Emily Schroeder, University of California - Santa Barbara
Steven V. Nystrom, University of Florida
Andrew (Bean) Getsoian, Ford Motor Company
Phillip Christopher, University of California Santa Barbara
David Hibbitts, University of Florida
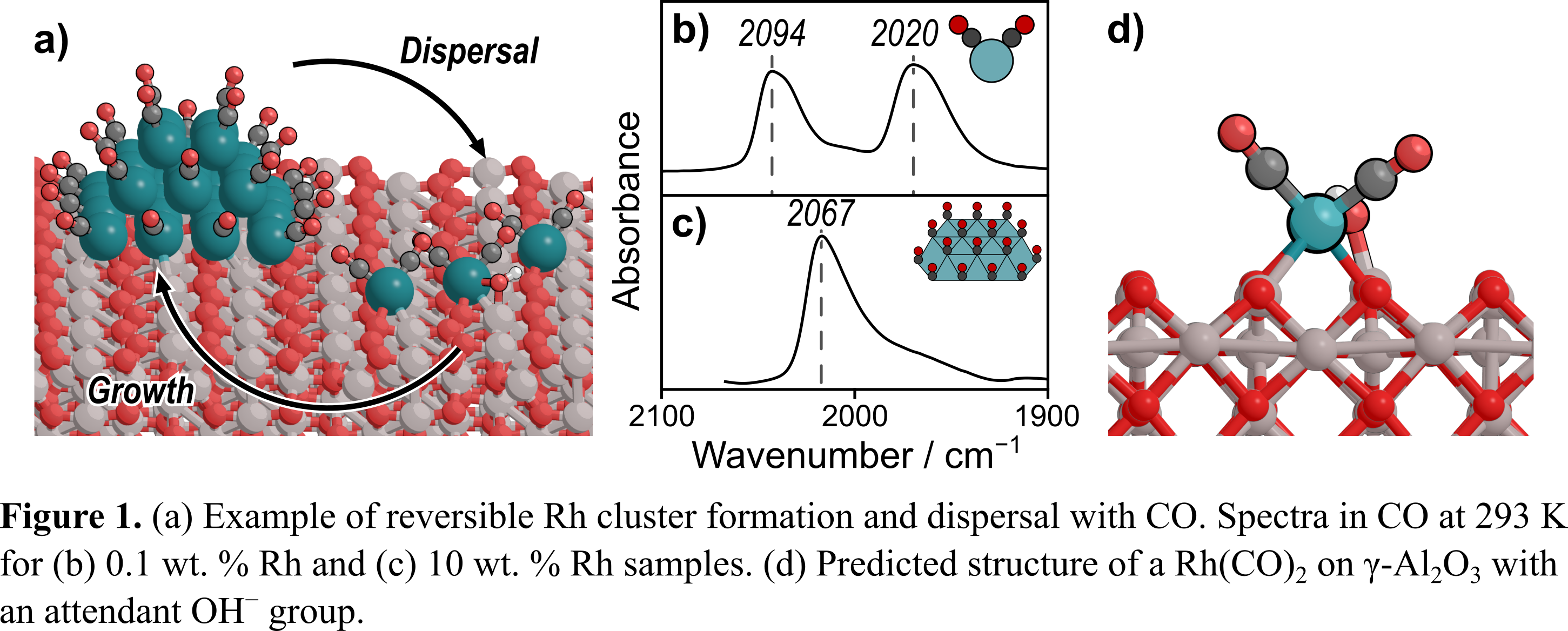
Despite the commercial use of three-way catalysts (TWC) for NOx reduction for over 40 years, the mechanism by which the Rh component of TWC reduces NO to N2, N2O, and NH3 remains a mystery [1]. Rh used in small weight loadings (<2%), characteristic of TWC, can reversibly restructure during catalysis between single-atoms and small nanoparticles (Fig. 1a) [2]; as such, well-defined materials are required to study the behaviors of these distinct forms of Rh catalysts. We use a combination of density functional theory (DFT) and CO probe-molecule IR to study the structure of atomically dispersed Rh on γ-Al2O3. When exposed to CO, an IR spectrum of a 0.1 wt. % Rh/γ-Al2O3 catalyst shows two predominant peaks at ~2090 and ~2020 cmâ1 at 293 K, corresponding to the symmetric and asymmetric stretches of a Rh(CO)2(Fig. 1b). These features contrast with our previous finding on a 10 wt. % sample, which exhibited one large peak at 2067 cmâ1 at 293 K, corresponding to atop-bound CO* on a Rh nanoparticle (Fig. 1c) [3]. Our DFT calculations indicate that these features correspond to a Rh(CO)2 coordinated to OH on the γ-Al2O3 support (Fig. 1d), with the Rh in a +1 formal oxidation stateâin agreement with earlier characterization work [4]. Critically, water can dissociatively adsorb on the surface of the γ-Al2O3 support, which alters the local environment around the Rh(CO)2, changing its stretching frequencies by up to ~10 cmâ1. Our work suggests that the first CO desorbs during temperature-programmed desorption from a partly hydrated γ-Al2O3, indicating that support hydration may be crucial in the operating behavior of TWC.
References
[1] Catalysis Reviews, 1994, 36, 433â457
[2] J. Am. Chem. Soc., 2013, 135, 1760â1771
[3] J. Phys. Chem. C, 2021, 125, 19733-19755