2022 Annual Meeting
(345b) Overcoming Challenges in Off-Stoichiometric Thermodynamics Modeling through Complementary Use of Experimental and First Principles Data: A Case Study of Ba1-XSrxFeO3-?
Authors
Steven Wilson - Presenter, Arizona State University
Ellen B. Stechel, Sandia National Laboratories
Christopher L. Muhich, University of Colorado at Boulder
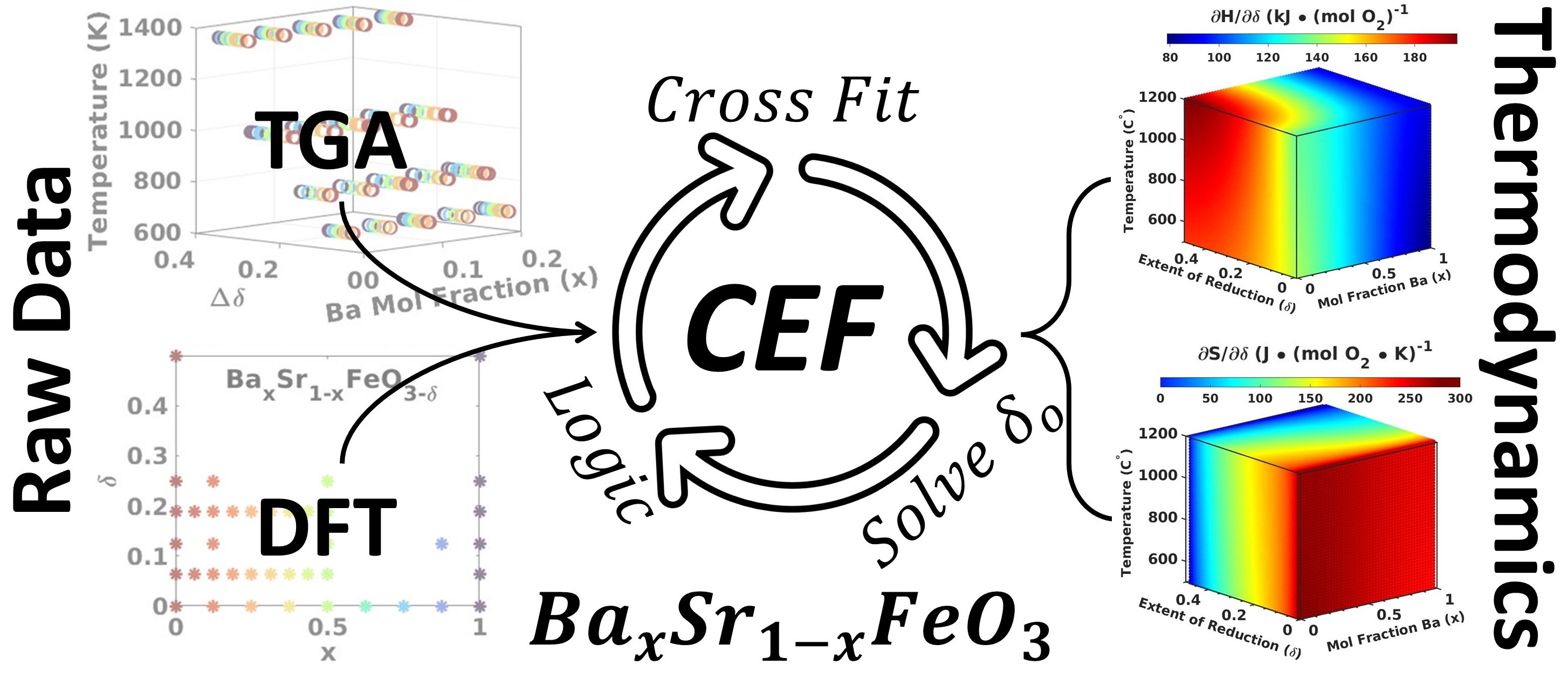
The compound energy formulism (CEF) is a powerful framework to describe the thermodynamics of metal oxides as a function of off-stoichiometry, temperature, and composition. The thermodynamic properties are crucial materials design attributes in metal oxide-based oxygen-exchange chemical processes. Despite the richness of information an accurate CEF model provides, a method to determine a unique and accurate fit for oxygen-exchange materials remains elusive. This contribution details a method for fitting the CEF model that overcome the current fitting challenges through three innovations: 1) the combination of density functional calculations with experimental data that delineates the enthalpic/entropic contributions to the Gibbs free energy; 2) a systematic determination of the important CEF model terms, removing thermodynamic predetermining human intervention; 3) a self-consistent solution of the starting oxygen off-stoichiometry (δ0) of thermogravimetric measurements. Thus, our method enables the reliable extraction of off-stoichiometric metal oxide thermodynamic properties and facilitates rapid materials compositional screening, and reliable process design of systems dependent on off-stoichiometric redox-active metal oxides. We apply this method to a BaxSr1-xFeO3-δ test case. We find by systematically examining the performance of the CEF model fit with and without each innovation that all three innovations are necessary for an accurate fit. We determined that reduction enthalpy is higher and more sensitive to off stoichiometry when the Sr fraction is large (139.5 and 185.3 kJ/mol O2 for SrFeO3 at δ=0 and δ=0.5, respectively vs. nearly constant 83 kJ/mol O2 for BaFeO3). However, the reduction entropy is mostly insensitive to Sr fraction, but highly dependent on δ