2022 Annual Meeting
(311g) The Influence of Channel Curvature on Targeted Particle Binding in Macroscopic Blood Flow
Methods: Channel were designed in Siemens NX to be 5 mm in diameter with radii of curvature of 25 mm, 37.5 mm, 50 mm, 100 mm, 200 mm, and straight. The channels were then printed on a Form 3 SLA printer. The prints were then coated in silicone rubber to create a negative of the channel. A monolayer of human umbilical vein endothelial cells was cultured in each model on a layer of crosslinked gelatin. Whole pigsâ blood was purchased from a local slaughterhouse and used as a model for human blood due to bulk volume needed for each flow experiment. RBCs were separated from whole blood samples using centrifugation with the plasma and white blood cells being discarded. The RBCs were then reconstituted in pH 7.4 phosphate buffer solution with 1% bovine serum albumin (flow buffer) at 40% hematocrit. Alternatively, for experiments not using RBCs, only flow buffer was used. Flow buffer and RBC solutions were circulated from a reservoir through a peristaltic pump into damper bottle then through the inflamed confluent model and finally back into the reservoir. The peristaltic pump was ramped up slowly to 450 mL/min over a 9-minute period to prevent damage of the living cells. After reaching full speed, Sialyl-Lewis A conjugated particles were injected into the reservoir and perfused for 1 hour. After perfusion the models were washed with phosphate buffer solution. The models were drained, sectioned, and then imaged using a stereomicroscope. Simulations in COMSOL were ran using the above flow parameters in the same geometry as the printed files and the assumed viscosity of blood.
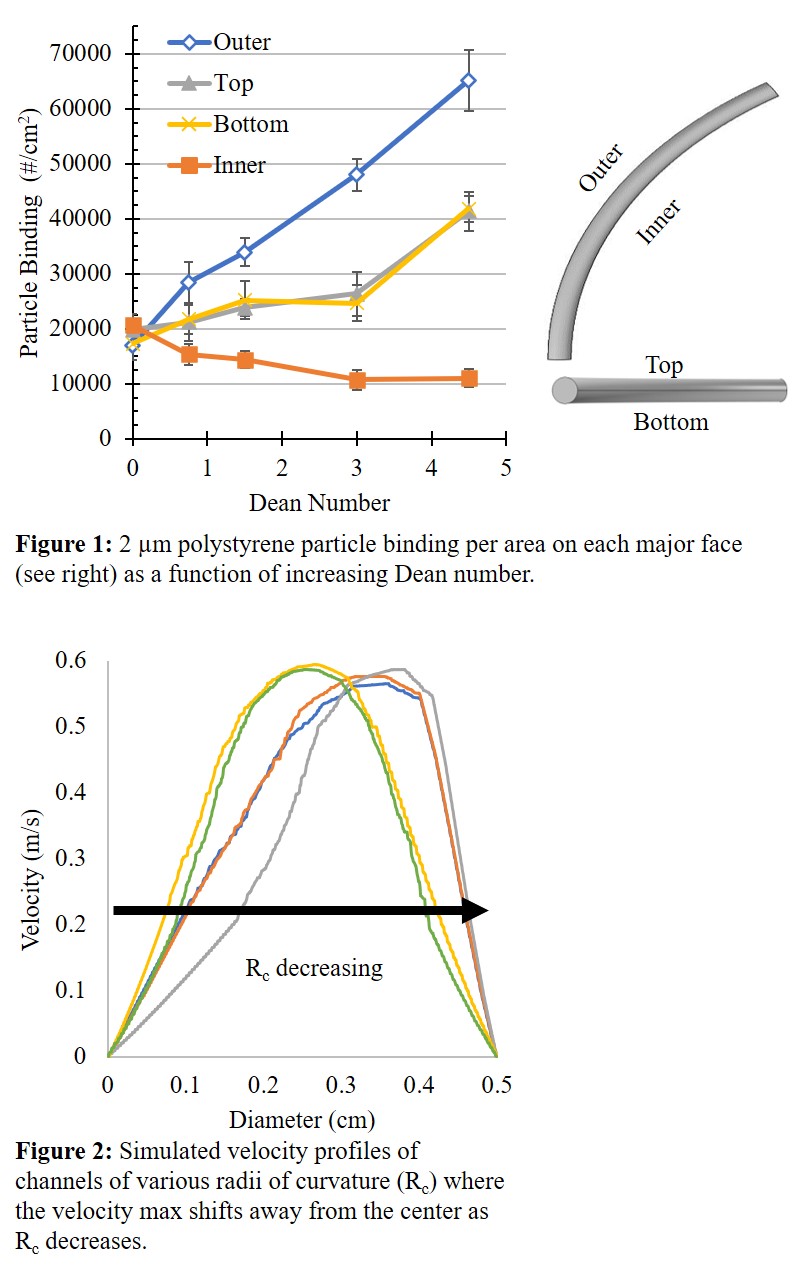
Result and Conclusions: This work highlights the effect of increasing curvature on the enhanced ability of targeted 2 µm particles to bind to the concave outer wall and the decreased ability for particles to bind on the convex inner wall, Figure 1. Further experimentation, has shown this trend to continue for that of the 500 nm particles and no RBCs cases, however, in both of these alternatives the particle binding efficacy (particle binding/total particles injected) was less than that of the 2 µm case. Despite being a low confinement system, channel curvature still plays a role in particle binding. Additionally, this highlights the importance of RBCs on the binding potential of in curved flow. Simulations of these channels show the velocity maximum shifting away from the center of the channel as radius of curvature decrease, Figure 2, and a simultaneous increase of shear stress on the walls the velocity maximum shifts towards. Likewise, COMSOL predicts the development of a secondary Dean flow that directs fluid towards the outer wall and away from the inner wall. This may be a contributing factor in the increased binding of the outer wall. By better understanding how particles bind in curved flow, VTCs may be better targeted towards large scale vascular plaques.