2022 Annual Meeting
(275e) Reactive CFD-DEM Simulations of Oxidative Coupling of Methane in a Gas-Solid Vortex Reactor
Authors
The gravitational fluidized bed reactor is the most applied technology for heterogeneously catalyzed processes due its higher heat and mass transfer efficiency compared to packed bed reactors. However, the constant drive for innovation in view of process intensification sparks interest in further improving the heat and mass transfer between catalyst pellets and the process gas stream. These transfer phenomena can predominantly be ameliorated by increasing the gas-solid slip velocities. An efficient way to achieve this goal is to operate in a centrifugal field instead of the gravitational field. Furthermore, a centrifugal field offers additional benefits such as the capability of handling larger flows in smaller and more energy-efficient reactors, thus contributing to process intensification.
Two categories of centrifugal fluidized bed reactors are distinguished based on the way momentum is transferred to the solids. This momentum can be supplied by a motor that makes the unit rotate. It can also be transferred from gas to solids in a static unit. In this static unit, also referred to as gas-solid vortex reactor (GSVR), gas is therefore introduced via tangentially inclined inlet slots.
Oxidative coupling of methane (OCM), a catalytically induced conversion of methane to ethane and ethylene in the presence of oxygen, is chosen as the chemical process of interest. When studying OCM in a packed bed reactor, hot spots are a predominant issue due to its high exothermicity. Also, good control over the residence time is necessary since ethane and ethylene can oxidize further to CO and CO2. The GSVR is thus an excellent choice for the OCM process since it possesses of both excellent heat transfer characteristics and a narrow residence time distribution.
In this study, oxidative coupling of methane in a static gas-solid vortex reactor (GSVR) is numerically investigated via an in-house developed CFD-DEM model accounting for heterogeneous catalytic chemistry.
Methodology
The four-way coupled CFD-DEM simulations presented in this work are performed with an in-house developed version of the open-source package CFDEMcoupling, which couples the CFD package OpenFOAM 8 with the DEM solver LIGGGHTS.
The conservation equations for mass, momentum and species are solved for a compressible fluid, with the inclusion of the particle volume fraction. An inter-phase momentum exchange term is present in the momentum conservation equation accounting for the gas-particle interaction forces, of which the largest contributor is the drag force. The drag force is calculated via the Gidaspow drag model. Gas-solid heat transfer is accounted for via the Gunn correlation. Gas-phase turbulence is modeled via the SST k-Ï turbulence model. Newtonâs law of motion is solved to track the displacement of individual catalyst pellets within the reactor chamber. The contact force between the solids is calculated based on the non-linear Hertz contact model.
Reactive simulations are performed with OCM micro-kinetic models for both a 4%Sn-2%Li/MgO and a 1%Sr/La2O3 catalyst. The micro-kinetic models are composed of 30 gas-phase and 10 surface species, covering 299 gas-phase and 26 surface reactions. The gas-phase kinetic network is derived by Stagni et al. [1], based on the CRECK kinetic mechanism [2]. The elementary surface reactions and their kinetic descriptors for the Sn-Li/MgO and Sr/La2O3 catalysts are determined by Kechagiopoulos et al. [3] and Alexiadis et al. [4].
In the presented work, the GSVR has a radius of 80 mm and a height of 15 mm. The process gas is fed to the reactor chamber through eight tangentially inclined inlet slots with a width of 1 mm and an inclination angle of 10°. The mesh, constructed for the complete 360° geometry, is composed of 425 920 computational cells.
It is well-known that the computational load during a CFD simulation increases tremendously when additional balance equations need to be solved for every specie in the gas-phase. Therefore, two chemistry speed-up algorithms are incorporated in the CFD framework, namely in-situ adaptive tabulation (ISAT) and particle agglomeration (PA). ISAT stores thermophysical compositions of both the gas-phase and solid surface in a multi-dimensional table along with the output of the chemistry solution step. Upon encountering an already stored thermophysical composition, the output is retrieved from the table, thus avoiding to execute a computationally demanding chemistry solution step. Next to speeding up the chemistry solution step, the number of systems, here equal to the sum of the number of computational cells and particles, to be solved during a single time step can be reduced using algorithms such as PA. In PA, an index is assigned to each catalyst pellet. This index is determined by the gas species concentration in the computational cell the particle is found and on its surface coverage. Based on this index, particles are distributed over a user-defined number of âbinsâ, much smaller than the total number of particles in the system. For each bin, the chemistry, which depends on the average gas phase concentrations and coverages of the particles inside the bin, is solved only once. Next, the result is mapped to each particle within said bin.
Results and discussion
Validation of chemistry implementation
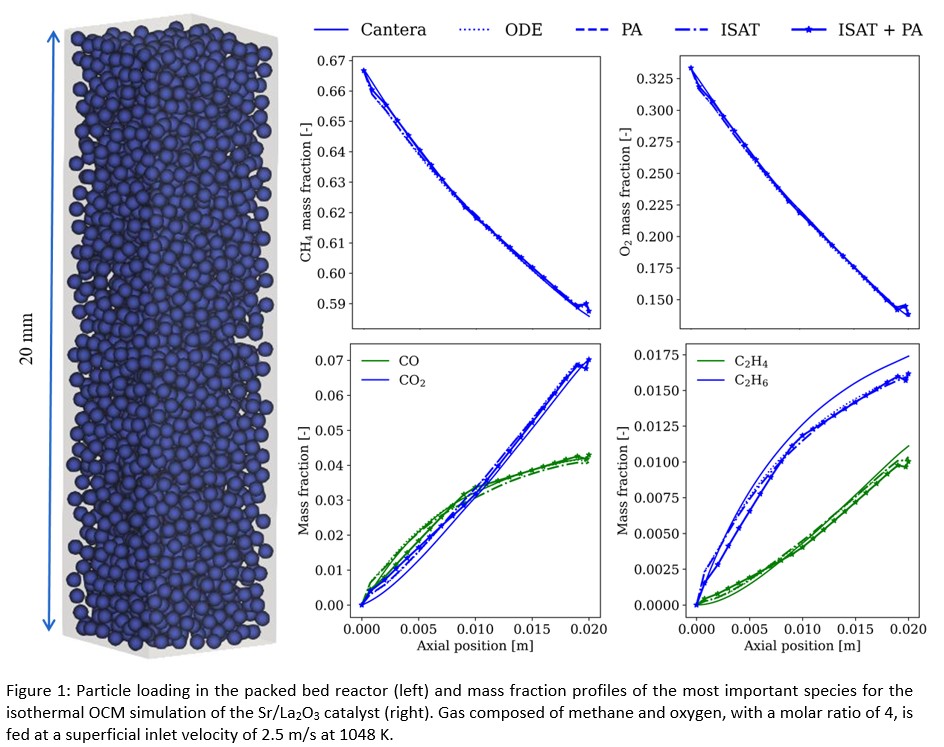
The implementation of the heterogeneous catalytic framework and of the aforementioned speed-up algorithms is first validated in a small packed bed geometry of 5 by 5 by 20 mm. Herein, positions of individual catalyst pellets are frozen in time. This allows to compare the CFD-DEM computational results one-on-one with the results obtained when using kinetic modeling software such as Cantera. Excellent agreement is found for both catalysts at different temperatures and inlet conditions, with and without applying speed up algorithms. Results for the Sr/La2O3 catalyst are shown in Figure 1. The aforementioned speed-up algorithms reduce the time necessary to determine the net species formation rate appearing in the species balance equations. When applying either ISAT or PA, a speed-up factor of 35 and 100 is obtained respectively. This speed-up factor increases to 110 when combining both methods.
GSVR simulations
CFD-DEM simulations of the GSVR geometry are performed using these chemistry speed-up algorithms. Several operational conditions, including methane-to-oxygen molar ratio in the process gas, inlet temperature, gas flow rate and loaded catalyst, are varied. The DEM formulation also allows to mix both catalysts together, thus combining the active properties of the Sr-based catalyst with the selective properties of the Sn-based catalyst. Both isothermal and adiabatic simulations are performed until a pseudo-steady state is reached. In order to limit the adiabatic temperature increase, the process gas is diluted with nitrogen during the adiabatic CFD-DEM simulations.
It is shown that the Sr-based catalyst is indeed more active than the Sn-based catalyst while the inverse holds for the selectivity towards C2-products. Methane conversion is found to increase at higher temperatures and lower gas flow rates, while C2 selectivity increases with increasing methane-to-oxygen molar ratio. However, this increase in molar ratio negatively impacts the methane conversion. Higher conversion is found in zones with higher catalyst volume fraction, i.e. upstream of a gas inlet slot. In these zones, catalyst buildup is observed due to the jet-like behavior of the inlet slots. The temperature of the entire catalyst bed is uniform for the adiabatic simulations, highlighting the intense heat transfer characteristics of the GSVR.
Conclusions
A reactive CFD-DEM modeling framework that is able to handle heterogeneous chemistry has been developed. Two methods which aid in reducing the computational cost are tested and compared. A model validation study is performed in a packed bed for the oxidative coupling of methane. Next, a study exploring the process intensification capabilities of a gas-solid vortex reactor is carried out. Isothermal and adiabatic simulations, using different catalysts and varying gas inlet composition and temperature, are performed. Even though OCM is highly exothermic, the adiabatic simulations show a uniform temperature profile in the catalyst bed. The gas-solid vortex reactor is thus considered an ideal candidate for intensification of processes such as the oxidative coupling of methane.
References
- Stagni, A., et al., The role of chemistry in the oscillating combustion of hydrocarbons: An experimental and theoretical study. Chemical Engineering Journal, 2020. 385: p. 123401.
- Ranzi, E., et al., A Wide Range Modeling Study of Methane Oxidation. Combustion Science and Technology, 1994. 96(4-6): p. 279-325.
- Kechagiopoulos, P.N., et al., Oxidative Coupling of Methane: A Microkinetic Model Accounting for Intraparticle Surface-Intermediates Concentration Profiles. Industrial & Engineering Chemistry Research, 2014. 53(5): p. 1825-1840.
- Alexiadis, V.I., et al., Oxidative coupling of methane: catalytic behaviour assessment via comprehensive microkinetic modelling. Applied Catalysis B: Environmental, 2014. 150-151: p. 496-505.