2022 Annual Meeting
(168c) Modulating the Differentiation of Human Neural Stem Cells in 3D Contexts By Varying Hyaluronic Acid Chain Length
Authors
In this study we used human neural stem cells (iHNSCs, Cell Applications) as the cell source. The cells were expanded according to the providerâs protocol using growth media supplemented with B27, basic fibroblast growth factor (bFGF), endothelial growth factor (EGF) and heparin. Cells were expanded and then divided into two groups: one group was encapsulated in the mIPNs and the other group was pre-differentiated for 5 days in neural differentiation media on plates coated with collagen I before being encapsulated. The mIPNs were composed of varying levels of hyaluronic acid (HA) high and low molecular weight (HA-HMW and HA, LMW), collagen type I (col I), and poly(ethylene glycol) diacrylate (PEGDA). The cells were collected and encapsulated based on a procedure previously developed by our laboratory1.The cells in the mIPNs were cultured at 37°C and 5.0% CO2 for 14 days in different differentiation media. Constructs were mechanically characterized using dynamic mechanical analysis (DMA) on a TA Instruments 3100 mechanical tester.
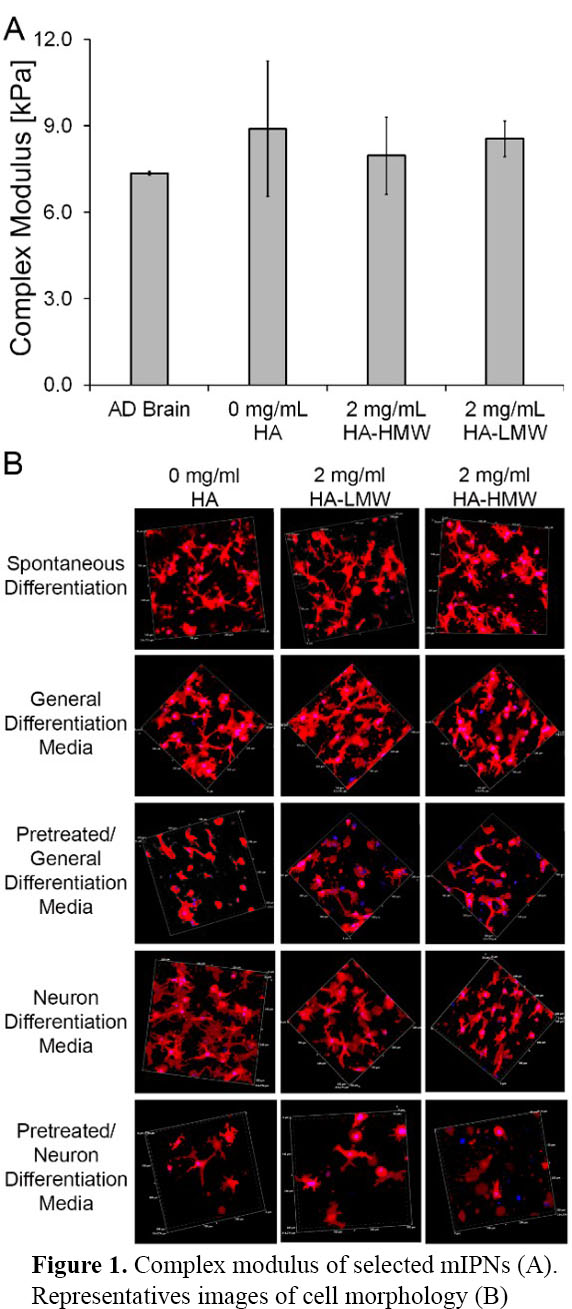
Mechanical data demonstrated that different mIPN formulations containing HA-HMW or HA-LMW were fabricated to closely match the complex modulus of human brain cortex tissue (Figure 1A). Initial qualitative assessment of cell morphology using Laser Confocal microscopy revealed that cell morphology was modulated by both pre-differentiation conditions and the presence of HA of different chain lengths. It appears that long HA chain length limits the spreading of cells in 3D contexts (Figure 1B). Gene and protein expression profiles are being analyzed to characterize the differentiation stage for each experimental group.
The proposed bioinspired mIPNs displayed complex modulus within the range of mechanical performance of human cortex tissue while displaying distinct chemical composition. The spreading of iHNSCs in our 3D scaffolds appears to be a function of HA content, HA molecular weight and pre-differentiation conditions.
Funding: The Alzheimerâs Association and Alzheimerâs Texas (AARGD-17-531470), McNair Scholars Program, Semmes Distinguished Scholars in Science Scholarship Fund and the Murchison Fellowship