2022 Annual Meeting
(139a) Development of a Single-Tablet-Scale Direct Compression Process for the on-Demand Production of Personalized Tablets
Authors
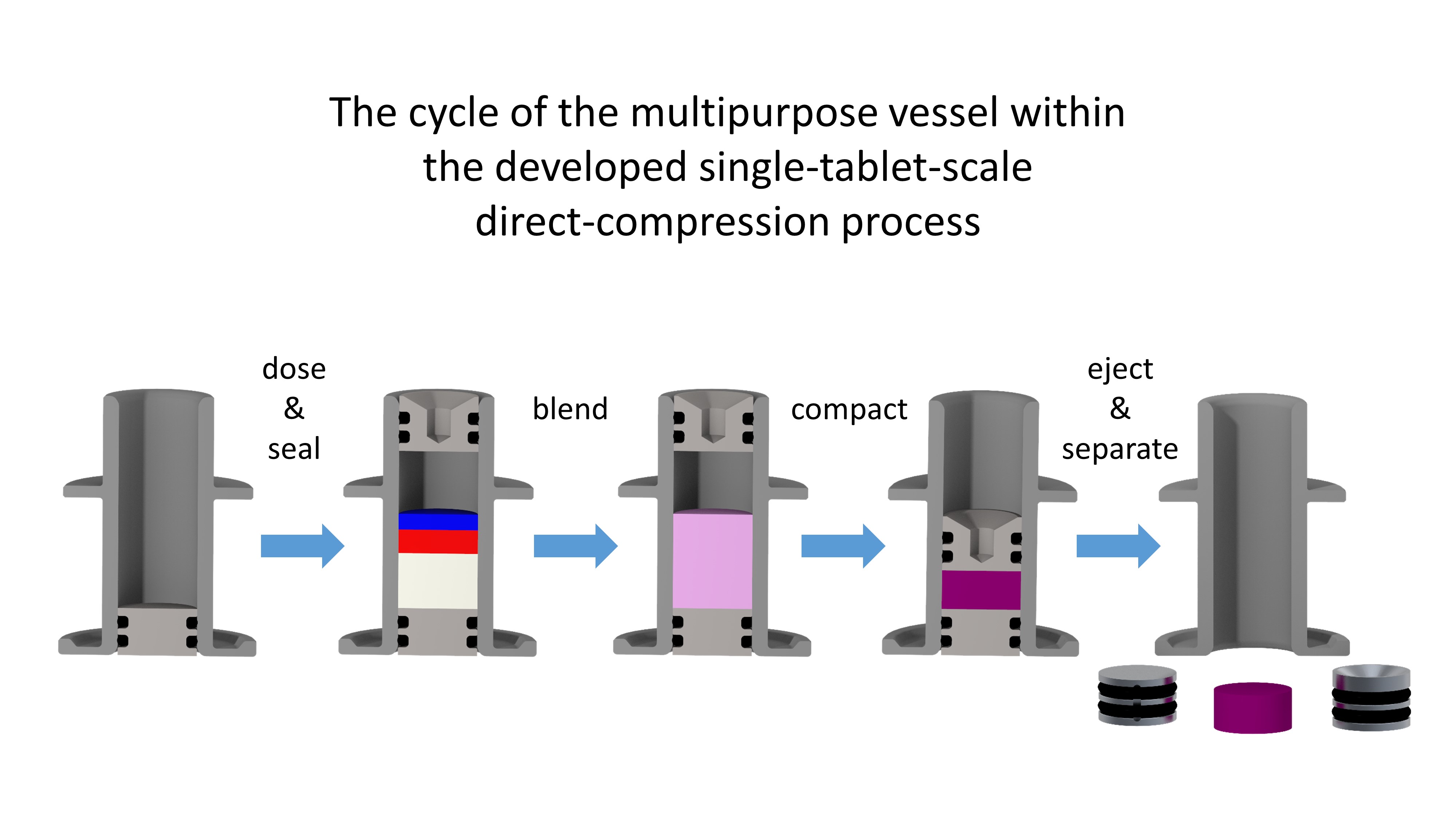
Dosing was realized using a device based on a volumetric microfeeder (1) which was combined with a weighing cell to provide gravimetric feedback. This facilitated the implementation of a control strategy to enable dosing of various pharmaceutical powders in the mg-range. The vessel used to collect the powder in the dosing step is subsequently sealed and used as the mixing container and finally as compaction die. Consequently, the powder masses dosed in the first step are exactly the amounts found in the final dosage forms, as there is no powder transfer or equipment entering or leaving the vessel. Therefore, blending needs to take place inside a sealed vessel having the dimensions of a compaction die (i.e. 10 mm in diameter and 20 mm in height). To solve this task, we used vibratory mixing as this was identified as a promising approach to blend small amounts of pharmaceutical cellulose powders to a satisfactory degree of homogeneity within less than 3 seconds (2). Compaction was done using a single punch tablet press consisting of an electric linear actuator equipped with a load cell and a mechanism to separate the tablet from the die.
To validate the capability of this process to produce high quality tablets, first each process step was investigated isolated. To do so, the dosing and mixing processes were investigated using different excipient and API powders evaluating dosing performance and blend homogeneity qualitatively and quantitatively. Finally, model tablets containing 200 mg of API (Ibuprofen), 190 mg of microcrystalline cellulose, and 10 mg of a disintegrant (crospovidone) were produced using the developed setup. These tablets were analyzed for their Critical Quality Attributes (CQAs), namely the API content, tensile strength, and dissolution profile.
The results regarding the dosing performance show the capability of dosing various powders largely independent of their flow characteristics within a variability of ±10% for masses down to 20 mg. Below that dose, cohesion and particle size decrease the dosing performance. Concerning the mixing process, vibration parameters were found allowing to blend different systems consisting of one coarse material (d50 > 100 µm) and a fine material (d50 < 50 µm) or two coarse materials within 5 seconds to a satisfactory homogeneous state. Challenges were identified for systems consisting solely of fine material. Finally, the investigations on the produced Ibuprofen tablets revealed a mean API content of 101.21 ± 0.93 % of the desired amount and a tensile strength of 1.00 ± 0.04 MPa. In dissolution testing 87.2 ± 3.3 % of API were in solution within 15 minutes meeting the USP criterion for immediate release Ibuprofen tablets (80 % in 60 min (3)).
We therefore conclude that the developed setup is a valuable platform for manufacturing personalized tablets and to produce tablets on demand from a limited number of ingredients. Future work will focus on resolving limitations of dosing as well as in blending of cohesive powders. Furthermore, additional model systems will be tested to investigate the operation space of the developed platform.
References
- M. O. Besenhard et al., Micro-feeding and dosing of powders via a small-scale powder pump. International journal of pharmaceutics. 519, 314â322 (2017), doi:10.1016/j.ijpharm.2016.12.029.
- A. Kottlan, B. J. Glasser, J. G. Khinast, Vibratory mixing of pharmaceutical powders on a single-tablet-scale. Powder Technology. 387, 385â395 (2021), doi:10.1016/j.powtec.2021.04.040.
- USP, Ibuprofen Tablets. In: USP29-NF24 .