2022 Annual Meeting
(122k) Dual Functional Catalysts for Cracking of Light Cycle Oil into Monoaromatics Using a Hydrogen Donor
Authors
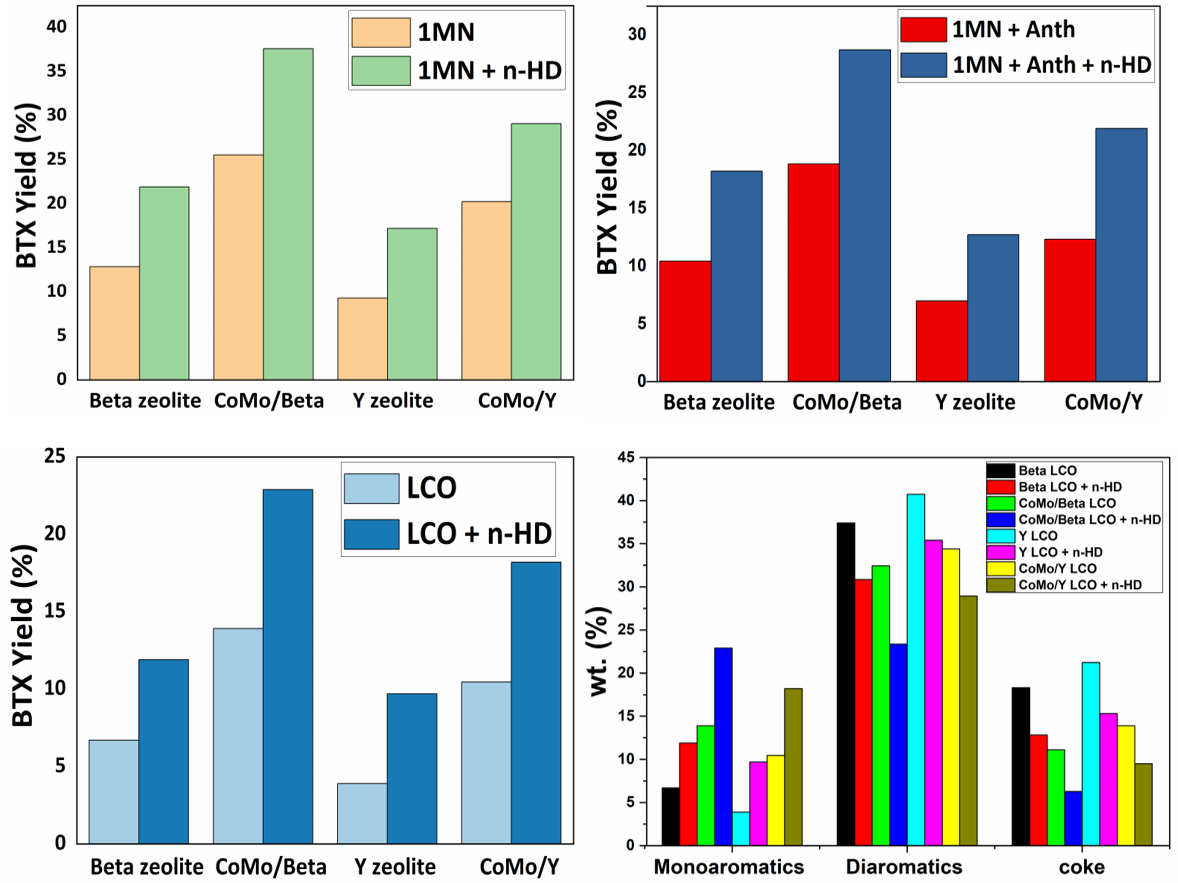
Globally, declining growth for fuels and rapid growth for petrochemicals has led oil refiners to look alternatives for converting crude to chemicals. In such a scenario, transformation of low value and low demand fuels to high value and high demand petrochemicals is highly benign. Light cycle oil (LCO) is obtained as a middle distillate in the diesel cut of the fluidized catalytic cracking (FCC) unit of petroleum refineries. Di and tri aromatics are the predominant molecular species in light cycle oil (LCO), that need to be converted to monoaromatics. However, conversion of LCO under FCC conditions is very low and remains least explored in the literature. The focus of this work is the conversion of diaromatics present in LCO to monoaromatics such as benzene, toluene, and xylene (BTX) under FCC conditions. Catalytic cracking of LCO and its model compounds was performed over monometallic (Co, Mo) and bimetallic (CoMo) catalysts supported over Beta and Y zeolite. Instead of hydrogen, n-hexadecane was used as the hydrogen donor under FCC conditions, which has the potential to reduce the process costs. The catalysts were synthesized by co-impregnation, and their performance was evaluated in a fixed-bed micro activity testing (MAT) reactor setup at 500oC and 1 atm. CoMo/Beta catalyst resulted in the maximum monoaromatics yield of 32.7% at 73.8% conversion of LCO in the presence of n-hexadecane, whereas in the absence of n-hexadecane, the corresponding monoaromatics yield was 25.3% at 48.6% LCO conversion. Thus, n-hexadecane serves as an effective hydrogen donor to promote the hydrogen transfer reactions and results in a higher yield of the desired product. The amount of coke also decreased in the presence of n-hexadecane. The monoaromatics yield for various catalysts followed the order: CoMo/Beta > CoMo/Y > Mo/Beta > Co/Beta > Mo/Y > Co/Y > Beta > Y, whereas the coke and diaromatics formation followed the reverse order, with CoMo/Beta resulting in the minimum coke and diaromatics formation . The improved catalytic activity of the bimetallic catalysts as compared to monometallic catalysts and commercial zeolites is attributed to the synergistic effect between Co and Mo. Metallic dispersion and interaction of Co and Mo with the support was confirmed using XRD, Raman, FTIR, UV-DRS, FE-SEM, HR-TEM, and XPS.