2020 Virtual AIChE Annual Meeting
(645d) Insight into Amorphous Solid Dispersion Dissolution Behavior: Laser-Diffraction and Raman Spectroscopy Holding Hands
Authors
Introduction
Formulation of Biopharmaceutics Classification System (BCS) class II and IV drugs into amorphous solid dispersions (ASDs) is a promising strategy to increase both the apparent aqueous solubility and bioavailability of these drugs. Although drug release from ASDs is still poorly understood, it has been suggested that drug release from ASDs is polymer-controlled at low drug loadings, whereby both drug and polymer release at the same rate (congruently) [1]. However, in contrast, when a higher drug loading is reached, which differs for different drug-polymer combinations, an abrupt decrease in drug release rate may be observed which may be explained by a switch to drug-controlled release, whereby release of drug and polymer is incongruent [2].
In the present work, it is intended to further understand the dissolution performance of different ASD formulations by following the dissolution rate of ASD particles using a laser-diffraction methodology. Furthermore, it will be also studied the possible drug enrichment on the partially dissolved ASD particles by Raman. Intrinsic dissolution was also applied to measure the dissolution rate of the API from the ASD matrix. For this study several ASDs were prepared with two grades of HPMC-AS having different ratios of succinoyl:acetyl groups (L and M) by spray-drying. A BCS class II drug will be used as a model drug.
Materials and methods
Production of ASDs
Amorphous solid dispersions were produced by spray-drying using a mixture of methanol and dichloromethane at 1:4 ratio (w/w), respectively, as solvents. The ASDs were produced at different Drug:HPMC-AS grades (L and M) and at different drug loads (15% and 35 % w/w). For these experiments, a Buchi Mini Spray Dryer B-290 was used with a two-fluid nozzle in closed loop. The spray-dried samples were further dried to remove any residual solvents in a vacuum drying oven at 40 ºC.
Physical characterization of ASD
Amorphous solid dispersions were characterized by X-Ray Powder Diffraction (XRPD) to confirm their amorphous nature. Morphology was studied by scanning electron microscopy (SEM) and particle size distribution was evaluated by Laser Diffraction (Sympatec).
ASDs and API dissolution performance
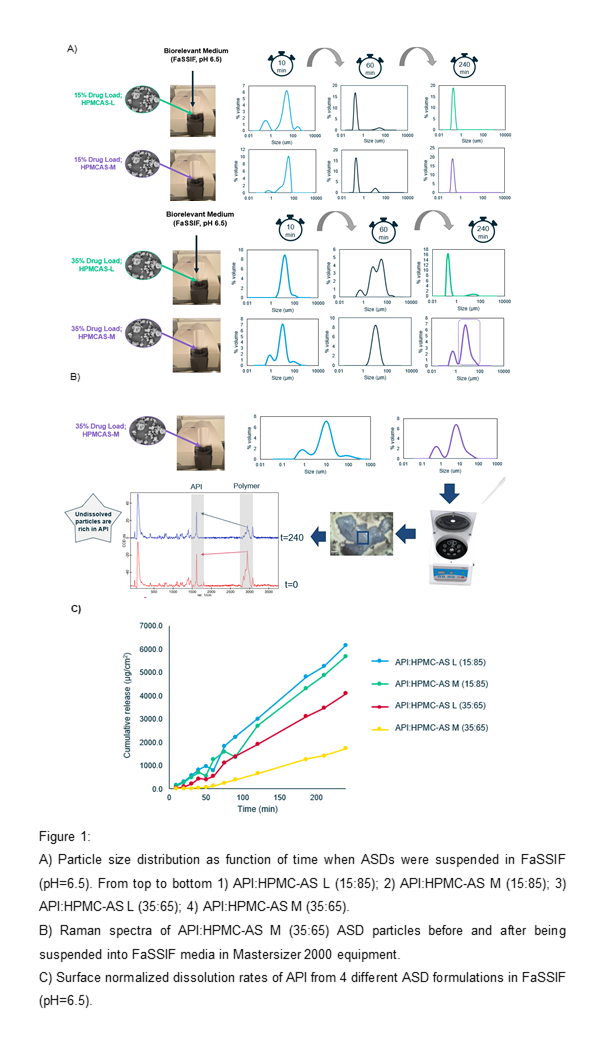
To get an insight into ASDs disintegration/dissolution kinetics, these ASDs particles were added to the dispersion unit of Mastersizer 2000 containing 150 mL of FaSSIF (biorelevant dissolution media). 86 mg and 200 mg of each ASD with 35 wt.% and 15 wt.% drug loads, respectively, were added to this dispersion unit to obtain 0.2 mg/mL as a drug target concentration. Particle size of ASDs was monitored for 4 hours. The reduction of particle size of ASDs during dissolution and colloidal formation was compared with the dissolution rate of the API released from these ASD particles. The drug concentration over time was measured by HPLC.
Results
ASD prepared by spray-drying showed an amorphous nature with no diffraction peaks from crystalline molecule. The particle morphology of the produced ASD was also analyzed by SEM. All the ASDs presented a shriveled morphology (raisin-like particles) which is characteristic of slower spray-drying kinetics. The particle size distribution was similar between different amorphous solid dispersions.
In order to monitor the dissolution performance of the four ASD, the different formulations were suspended in FaSSIF (pH 6.5) in the dispersion unit of the Mastersizer 2000.
In all ASD formulations it was possible to see the appearance of a colloidal population with a submicron size (< 600 nm) after the 240 minutes (Figure 1, A). ASD dissolution showed to be slower in formulations containing higher API loading, since after 60 minutes it is still possible to observe ASD particles in suspension. Itâs worth mentioning that for formulation - API:HPMC-AS M (35:65) - a full disintegration of ASD did not occur even after 240 minutes.
Raman imaging was performed to the API:HPMC-AS M (35:65) ASD before and after being submitted to the experiment in the Mastersizer equipment. Since part of this sample was not dissolved after 240 minutes, an aliquot was taken from the dispersion unit and centrifuged prior Raman analysis. The intensity of one specific Raman band of the API (1605 cm-1) and the Polymer (2943 cm-1) were monitored in ASD particles before and after the experiment. Results showed that the ratio of 2943 cm-1/1605 cm-1 bands is above 1 (red spectra, Figure 1, B) in this ASD particles before being suspended into FaSSIF. However, this ratio showed to invert after the experiment, i.e, the polymer specific band at 2943 cm-1 seems to reduce faster than API specific band suggesting an absence of a concomitant dissolution of both elements when this ASD is suspended in FaSSIF. This is consistent with a surface drug enrichment phenomenon [2], suggesting faster dissolution of the polymer in relation to the API.
Intrinsic dissolution confirms that the dissolution rate of ASDs is aligned with the dissolution of ASDs particles observed in laser-diffraction experiment. It is possible to note that formulations containing 35% of API present slower dissolution rates than the ones containing 15% of API. Again, API:HPMC-AS M (35:65) formulation showed to have the lowest performance (Figure 1, C), which seem to be related with a drug enrichment of ASD particles in this particular formulation.
Conclusions
The results presented in this work indicate that dissolution of ASD particles (by laser-diffraction) give an important insight on the API dissolution rate. In detail, it was interesting to note that formulations which showed to disintegrate/dissolve faster leading to a stable colloidal suspension in laser-diffraction test were also the formulations with the highest and fastest API dissolution rate. This proves that their improved performance is related with the ASD dissolution mechanism. The faster dissolution of ASD particles in 15% drug load formulations suggests possible polymer-controlled dissolution which is associated with low drug loadings formulations [2]. The Raman analysis of API:HPMC-AS M (35:65) particles after being suspended/stirred in FaSSIF for 240 min suggested a faster dissolution of the polymer in comparison to the API, leading to particles enriched in API. This explains the slowest dissolution rate observed in this formulation. Therefore, it can be concluded that API dissolution performance is impacted not only by the drug load but also by the HPMC-AS grade. As a future work, the different hydrogen bonding between the API and the 2 different grades of HPMC-AS with varying ratios of succinoyl:acetyl groups will be explored. This study also shows that laser diffraction and Raman spectroscopy pose as orthogonal techniques to understand the dissolution mechanisms of ASDs.
REFERENCES
Tres F, Posada MM, Hall SD, Mohutsky MA, Taylor LS. Mechanistic understanding of the phase behavior of supersaturated solutions of poorly water-soluble drugs. Int J Pharm. 543(1-2): 2018
Saboo S, Kestur US, Flaherty DP, Taylor LS. Congruent release of drug and polymer from amorphous solid dispersions: insights into the role of drug-polymer hydrogen bonding, surface crystallization and glass transition. Mol Pharm. 17(4): 2020.