2020 Virtual AIChE Annual Meeting
(628d) Enhanced CH4 Conversion over Pt+Pd/Al2O3 + Mn0.5Fe2.5O4 Spinel Catalyst: Impact of Oxygen Storage Material
Authors
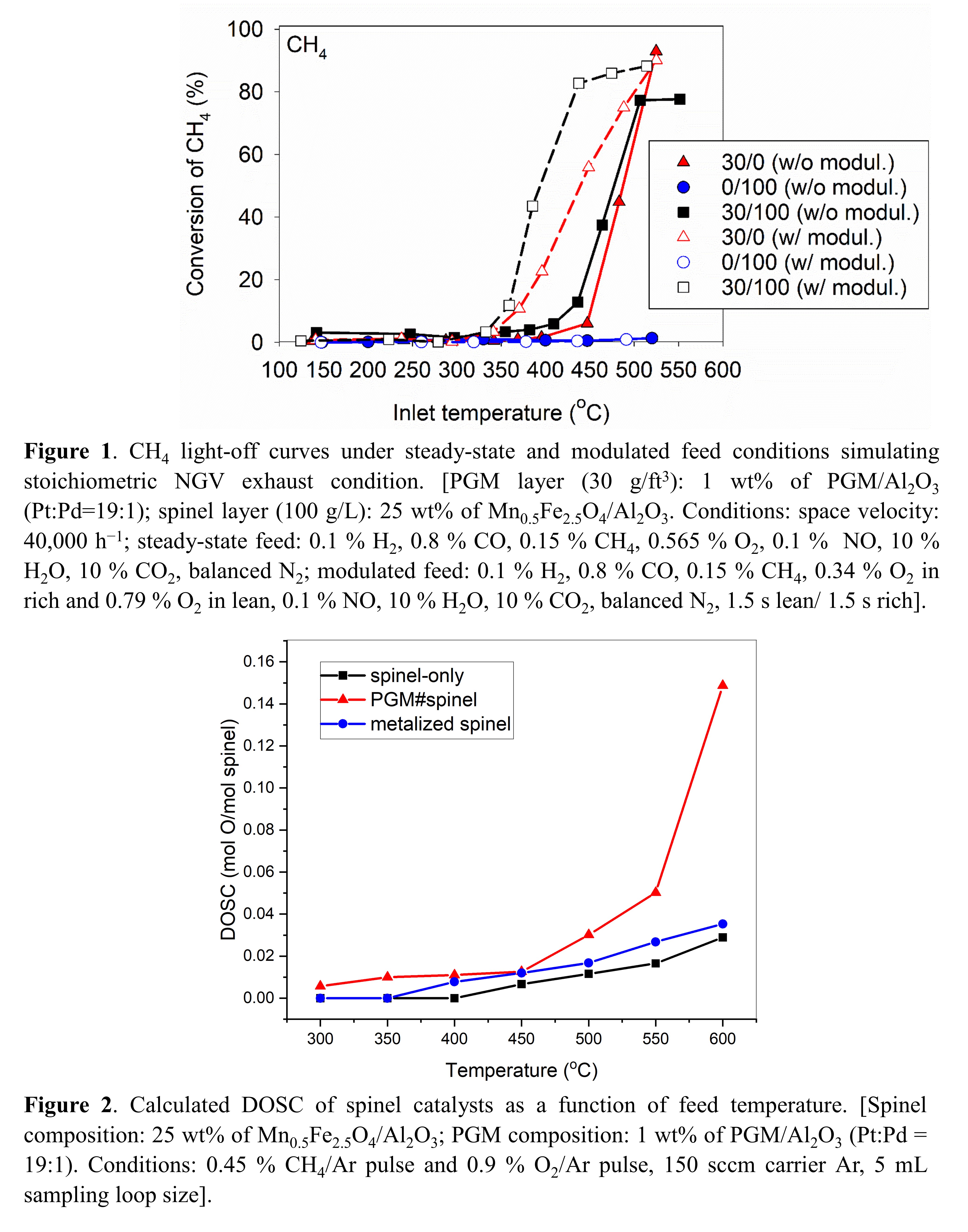
Dual-layer [â30/100â: 30 g PGM/ft3 monolith, 100 g spinel (25% on Al2O3)/L monolith], single-layer (30/0, 0/100) monolith catalysts and several powder samples were provided by CDTi for flow experiments and DOSC measurements. The powder mixture of PGM and spinel (PGM#spinel) simulated the dual-layer sample, whereas the metalized spinel sample had close contact between PGM and spinel.
Figure 1 shows the combination of lean/rich feed modulation and spinel addition allowed for improved CH4 conversion [2]. The enhancement is due in part to the high DOSC of PGM#spinel. DOSC comparison on PGM#spinel and metalized spinel suggests PGM proximity to spinel is detrimental, Figure 2. Spinel addition also limits steady-state CH4 conversion above 500ËC, Figure 1. This is tentatively attributed to differences in reforming activity, which dominates at high CH4 conversion with O2 depletion. TAP experiments are used to resolve the mechanistic intricacies between oxygen storage, oxidation and reforming.
Our study provides fundamental and practical insight into the function of spinel on CH4 conversion, which paves the path for optimizing catalyst formulation and operation of NGV emission control.
References
- S. Golden, Z. Nazarpoor, M. Launois, R-F. Liu, P. Maram, Society of Automotive Engineering. SAE 2016-01-0933 (2016).
- S. Kang, K. Karinshak, P.W. Chen, S. Golden, M.P. Harold (in press), Catalysis Today, (2020).