2020 Virtual AIChE Annual Meeting
(622e) Synthesis, Characterization and Functionalization of Chitosan and Gelatin Type B Nanoparticles with Cell-Penetrating Capabilities
Authors
Even if the nanocarriers are able to penetrate cells, they might be intracellularly trafficked via endocytosis, a process in which the cell engulfs extracellular materials into the cell by invagination of the cellular membrane forming endosomes [5]. Endocytosis can be classified into phagocytosis or pinocytosis. The former is a mechanism for large solutes while the latter is for liquids and small particles. Nanoparticles with size from 250 nm to 3 μm have shown to enter via phagocytosis, while the ones with a size range of 120 nm to 150 nm are internalized via clathrin or caveolin-mediated endocytosis [5]. This specialized route uses ligands that bind to specific receptors in the membrane, which are then engulfed within clathrin-coated vesicles. In any case, the formed endosomes eventually reach lysosomes where degradation enzymes break down the contents. For this reason, to achieve high distribution levels of the drug, the delivered nanocarrier must be able to achieve endosomal escape once inside the cell [5]. Internalization is also a function of surface charge, coating type, and elastic modulus. For instance, soft nanomaterials (e.g., liposomes and hydrogels) tend to have faster and more effective internalization than the hard ones (e.g., metallic oxides) [5].
Studies show that polymeric nanoparticles can be engineered to induce endosomal escape by destabilizing and disrupting endosomal membranes. Moreover, polymers with a buffering capacity can also inhibit the acidification of the endosome, thereby resulting in an influx of chloride counterions and water molecules that increases the pressure and ultimately lyses the endosome. Here, we propose to synthesize two types of polymeric nanoparticles, gelatin type B and chitosan to subsequently functionalize their surfaces with the translocating peptide Buforin II such that the buffering capacity of polymers is synergistically combined with potent endosomal escape moieties [6]. Gelatin B was selected due to its low-cost and superior biodegradability and biocompatibility, while on top of these attributes, chitosan has been reported to have remarkable mucoadhesive and antibacterial properties [7]. Additionally, we will explore the impact of the molecular weight of the polymers for the preparation of chitosan nanoparticles as it has been reported that, depending on this parameter, it is possible to achieve different endosomal escape levels [8].
The gelatin type B nanoparticles were synthesized via the desolvation method where acetone was incorporated as a desolvating agent that is added to an aqueous solution with the material to dehydrate it. As a result, gelatin undergoes a conformational change to form spirals that are crosslinked to form the nanoparticles [9]. Briefly, 200 mg of type B gelatin were dissolved in 10 mL of distilled water followed by dropwise addition of 10 mL of acetone. Two phases were then formed and the supernatant was discarded. The precipitate was redissolved in 10 mL of an aqueous HCl solution (pH 3) under constant stirring (10,000 RPM). Then, an additional 30 mL of acetone and an aqueous solution of 100 µL of 25% (v/v) glutaraldehyde were added to form the nanoparticles. Subsequently, the mixture was left under stirring for 30 minutes to ensure proper crosslinking and the NPs were then centrifuged and thoroughly washed with type I water several times. Finally, the nanoparticles were stored in PBS at 4 °C until further use [10].
The chitosan nanoparticles were synthesized by the ionic gelation method, which is based on the interaction between the positively charged amino groups of chitosan and the negatively charged groups of sodium tripolyphosphate (TPP) [11]. Briefly, Chitosan was dissolved in an aqueous solution of 1.25% (v/v) acetic acid. Then, an aqueous solution was formed at a concentration of 1.25 mg / mL maintained under stirring for 24 hours. After that, the pH was adjusted to 4.7 with a 1N NaOH solution and filtered with a vacuum filter unit equipped with a 0.45 µm PTFE filter. Subsequently, a TPP solution at a concentration of 0.56 mg/mL was added dropwise and kept under stirring for 30 minutes. Then, the nanoparticles were centrifuged and thoroughly washed with type I water several times. Finally, nanoparticles were stored in PBS at 4 °C until further use [11].
Buforin II was conjugated to the nanoparticles by pipetting 50μL of glutaraldehyde to activate the amine groups for one hour. Then, 1 mg of Buforin II was added and left under constant stirring for 48 hours. Excess glutaraldehyde was removed by centrifugation and thoroughly washing with type I water several times.
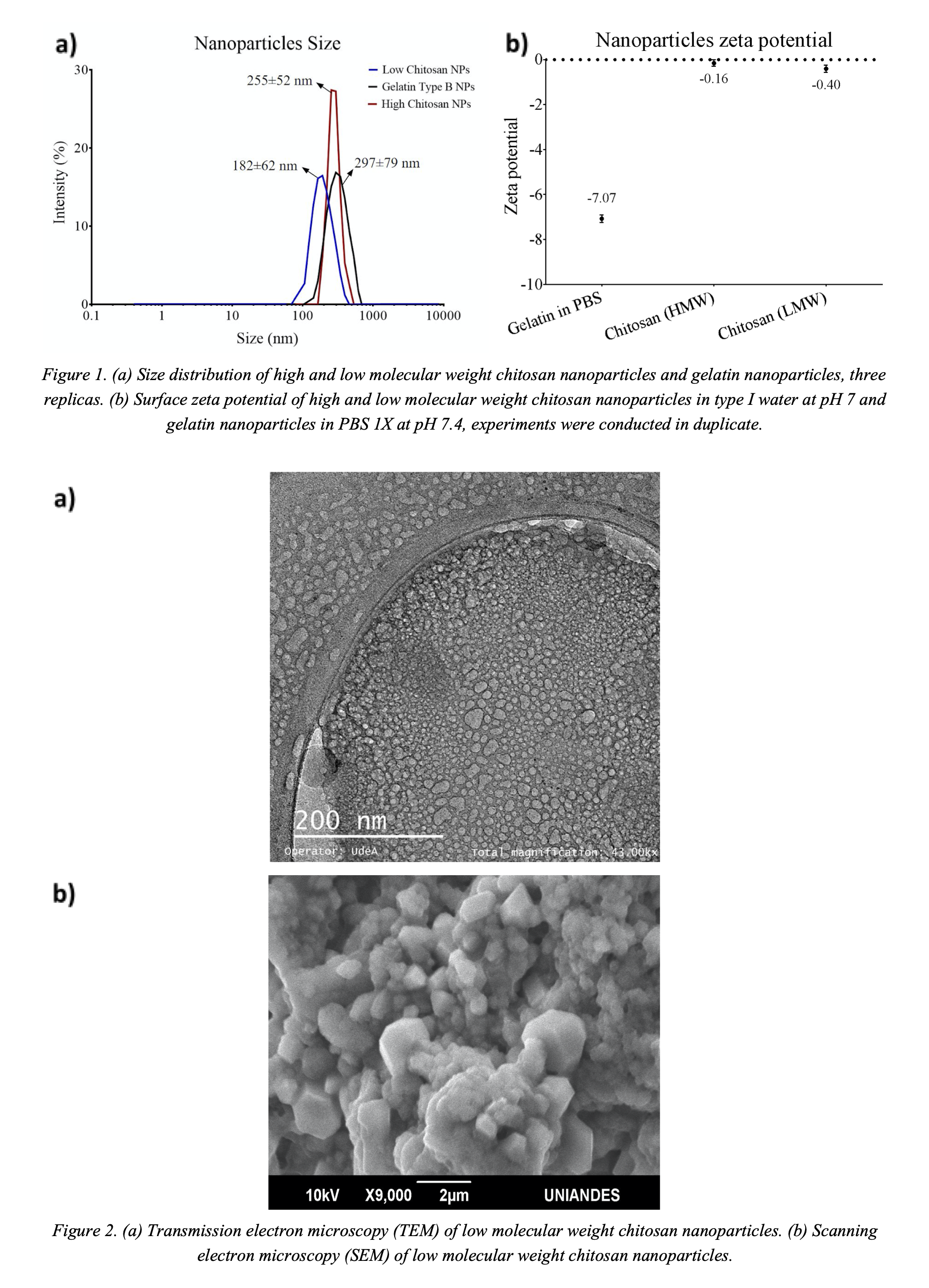
The obtained nanoparticles were characterized by Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM), confocal microscopy, transmission electron microscopy (TEM). The nanoparticles' size in solution and surface zeta potential were determined with a DLS instrument (Zetasizer Nano). Likewise, to determine the nanoparticles' endosomal escape, the nanoparticles were marked with rhodamine B and visualized via confocal microscopy. The FTIR confirmed the typical bands of the polymers and Buforin II after conjugation. Figure 1a shows the nanoparticles average diameters for gelatin (297±79 nm), chitosan of low molecular weight (182±62 nm), and chitosan high molecular weight (255±52 nm). The zeta potential for both low and high molecular weight chitosan nanoparticles approach neutrality (Figure 1b). This is problematic due to low colloidal stability. In contrast, the gelatin nanoparticles exhibited zeta potential of about -7 mV (Figure 1b). This is close to what is required for colloidal stability, i.e., ±10 mV. Figure 2a shows a TEM micrograph of the low molecular weight chitosan nanoparticles. This image confirms round-shape morphology and sizes in the range of 5-10 nm for individual particles. The SEM micrograph (Figure 2b) shows agglomerates of the low molecular weight chitosan nanoparticles. Similar results were found for the other polymeric nanoparticles.
References
[1] B. Devasier and K. Sanghyo, Polymer Nanoparticles for Smart Drug Delivery. Rijeka: InTech, 2014, pp. 257-310.
[2] W. Wang, K. Gaus, R. Tilley and J. Gooding, "The impact of nanoparticle shape on cellular internalisation and transport: what do the different analysis methods tell us?", Materials Horizons, vol. 6, no. 8, pp. 1538-1547, 2019.
[3] S. Dizaj, S. Jafari and A. Khosroushahi, "A sight on the current nanoparticle-based gene delivery vectors", Nanoscale Research Letters, vol. 9, no. 1, p. 252, 2014.
[4]J. Jeevanandam, A. Barhoum, Y. Chan, A. Dufresne and M. Danquah, "Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations", Beilstein Journal of Nanotechnology, vol. 9, pp. 1050-1074, 2018.
[5] P. Foroozandeh and A. Aziz, "Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles", Nanoscale Research Letters, vol. 13, no. 1, 2018.
[6] D. Elmore, "Insights into buforin II membrane translocation from molecular dynamics simulations", Peptides, vol. 38, no. 2, pp. 357-362, 2012.
[7] M. Farshbaf, S. Davaran, A. Zarebkohan, N. Annabi, A. Akbarzadeh and R. Salehi, "Significant role of cationic polymers in drug delivery systems", Artificial Cells, Nanomedicine, and Biotechnology, vol. 46, no. 8, pp. 1872-1891, 2017.
[8] S. Smith, L. Selby, A. Johnston and G. Such, "The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery", Bioconjugate Chemistry, vol. 30, no. 2, pp. 263-272, 2018.
[9]R. Donev, Advances in protein chemistry and structural biology. Amsterdam: Elsevier, 2015, pp. 169-221.
[10] B. Azimi, P. Nourpanah, M. Rabiee and S. Arbab, "Producing Gelatin Nanoparticles as Delivery System for Bovine Serum Albumin", Artificial Cells, Nanomedicine, and Biotechnology, vol. 18, no. 1, pp. 34â40, 2014.
[11] E. Carmona, T. Plaza, G. Recio-Sánchez and J. Parodi, "Generación de un protocolo para la sÃntesis de nanopartÃculas de quitosano cargadas con florfenicol a través del método de gelación iónica", Revista de Investigaciones Veterinarias del Perú, vol. 29, no. 4, pp. 1195-1202, 2018.