2020 Virtual AIChE Annual Meeting
(576a) Investigating Indirect Ultrasound on Particle Size, Shape and Nucleation of APIs through Process Intensification
Authors
Background
Particle engineering of active pharmaceutical ingredients (API) is an increasingly studied area of research & development. During drug substance manufacturing, particle formation typically originates from crystallisation which is a critical multiphase unit operation for the purification and separation of APIs. It is imperative that product properties such as particle size, shape and polymorphic form are well defined and well controlled as they affect a drugâs bioavailability, shelf-life and to effectively formulate the API into a finished drug product. [1]
However, achieving control over these properties is not necessarily met at the point of API crystallisation and isolation. Process intensified strategies through incorporation of ultrasound devices has shown wide interest in the last decade for manipulating particle properties during or immediately after the crystallisation step. For instance, the integration of ultrasound with crystallization allows for multipurpose functionality such as particle size & shape modification, [2] de-agglomeration, [3] scale-up, [4] accelerating the rate of crystallization kinetics and presents an overall more flexible approach to particle engineering. [5]
Ultrasound technology is commonly applied across several industries through insertion of a probe horn in direct contact with a liquid medium. For pharmaceutical crystallisation, this can be advantageous in process development especially when small seed particles <15 µm are preferred for controlling the crystal growth and thus, final product properties. [6] Whilst still a popular method, direct contact of the probe horn with particles has well reported limitations such as surface erosion, [7] substantial temperature deviations, [8] excessive noise in the vicinity of health and safety, rapid dissipation of energy transferred per unit volume and continuous operability. Therefore, it is worthwhile exploring alternative particle engineering strategies which can avoid the aforementioned limitations whilst ensuring the manufacturability and quality of the drug substance.
Case-Study
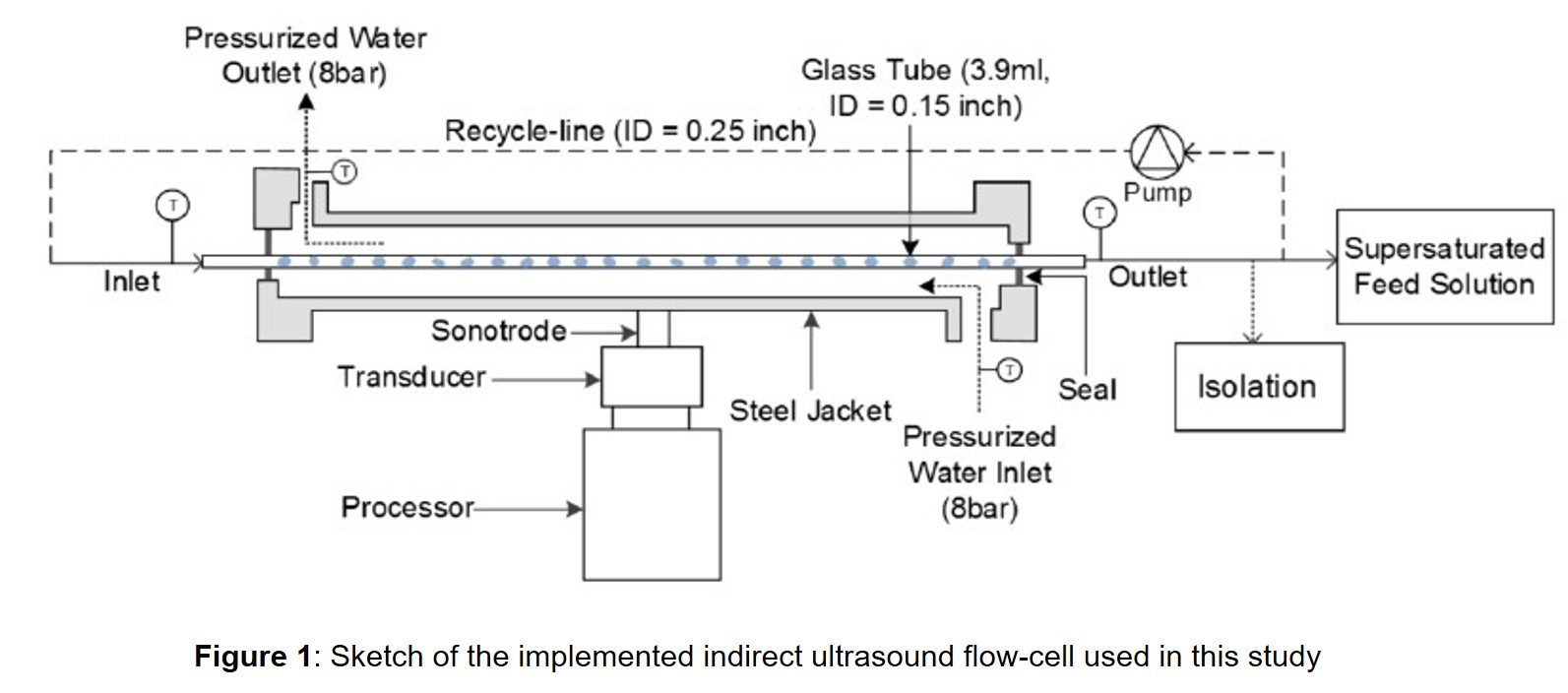
In this work, indirect ultrasound is explored as a viable and robust means for particle engineering of APIs. A semi-continuous platform which applies indirect ultrasound for inducing nucleation, particle size reduction and shape modification is experimentally investigated via process intensification. The process consists of an inline flow-cell which generates intense ultrasonic vibrations via electrical stimulation (Figure 1). These mechanical vibrations are then transferred to a glass tube which in turn sonicate the incoming process stream without direct contact.
Two APIs are selected for this study both of which have been found hard to nucleate with standard operating conditions under current industrial manufacturing processes. To establish key relationships between process parameters and product attributes, a series of isothermal desupersaturation experiments were conducted which monitored the impact of power amplitude (0, 20%, 40% and 80%) on inline count profiles, chord length distribution (CLD), particle size distributions (PSD) and solution concentrations. Nucleation rates affected from ultrasound impact were then extracted through population balance modelling as a proof-of-concept demonstration. To our knowledge, this is the first study to test and implement this indirect ultrasound flow-cell strategy on APIs for nucleation acceleration and control of particle attributes. The overall platform integration and ease of operation were additionally assessed for potential application in continuous manufacturing of pharmaceuticals.
References:
- Variankaval, N., A.S. Cote, and M.F.J.A.J. Doherty, From form to function: Crystallization of active pharmaceutical ingredients. 2008. 54(7): p. 1682-1688.
- Zeiger, B.W. and K.S.J.J.o.t.A.C.S. Suslick, Sonofragmentation of molecular crystals. 2011. 133(37): p. 14530-14533.
- Cote, A. and E.J.A.P.R. Sirota, CRYSTALLIZATION: the pursuit of a robust approach for growing crystals directly to target size. 2010. 13(7): p. 46.
- Kim, S., et al., Crystallization process development of an active pharmaceutical ingredient and particle engineering via the use of ultrasonics and temperature cycling. 2003. 7(6): p. 997-1001.
- Ruecroft, G., et al., Sonocrystallization: the use of ultrasound for improved industrial crystallization. 2005. 9(6): p. 923-932.
- Jiang, M., et al., Indirect ultrasonication in continuous slug-flow crystallization. 2015. 15(5): p. 2486-2492.
- Price, C.J., Application of Ultrasound in Crystallization (Sonocrystallization), in Engineering Crystallography: From Molecule to Crystal to Functional Form. 2017, Springer. p. 301-313.
- Zhang, Z., et al., Enhancement of crystallization processes by power ultrasound: current stateâofâtheâart and research advances. 2015. 14(4): p. 303-316.