2020 Virtual AIChE Annual Meeting
(574d) Simultaneous Activation of Methane (CH4) and Nitrogen (N2) in a Microwave-Enhanced Catalytic Reaction: A Combined DFT Modelling and Experimental Study
Authors
DFT and MKM simulations can be a very useful tool in elucidating heterogeneous catalyst behavior, especially for a reaction as complex as the one demonstrated in this work. This report investigates the feasibility of such a complex reaction using plane wave density function theory (DFT) and microkinetic modelling (MKM). The production trends and the catalyst behaviors are rationalized using these computational models and conformed experimentally. Further, an in-situ catalyst regeneration method is proposed based on the nature of the coke deposited on the catalyst surface. Lastly, the coked catalyst is regenerated in the microwave reactor to examine catalyst stability and reproducibility.
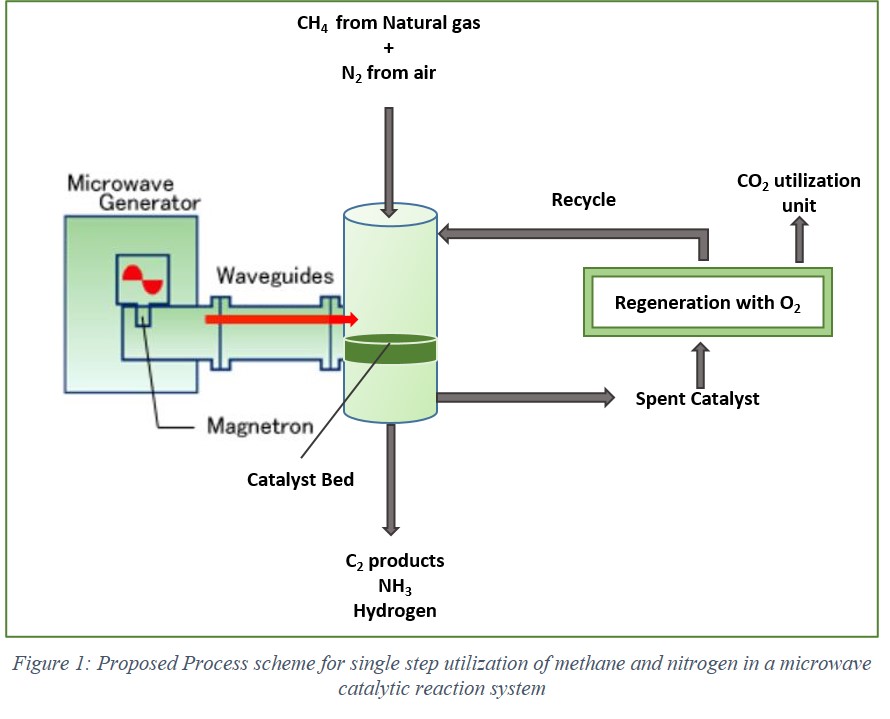
The co-production of ammonia, ethane, ethylene and acetylene demonstrated the activation of two highly stable molecules, CH4 and N2. The results from density functional calculations and micro-kinetics modelling predicted the reaction behavior at temperatures ranging from 573 K to 973 K and atmospheric pressure. The significant influences of terrace (111) and step (211) sites on K promoted Ru crystals were established in product formation and catalyst coking. NH3 formation occurs predominantly on the step (211) sites while C2 product formation can occur on both the sites. It was found that the elementary reaction steps leading to NH3 synthesis occurs due to the non-thermal effects of microwave irradiation while C2 products formed simultaneously are due to the thermal effects only. A reaction mechanism is postulated on the catalyst coking and validated experimentally by in-situ regeneration using H2 and O2.
In conclusion, this first of a kind reaction demonstrated in this study establishes the possibility of building a single-step small scale reaction system that has the potential of utilizing stranded natural gas resources in direct manner to produce value-added chemicals. The small-scale atmospheric pressure ammonia synthesis can not only lower the high energy requirements of the conventional production plants but also promote the use of the low-capacity and intermittent, renewable energy resources