2020 Virtual AIChE Annual Meeting
(55f) Novel Representation of Saturated Acyclic Hydrocarbon Chains and Its Application in Stochastic Reconstruction Model
Authors
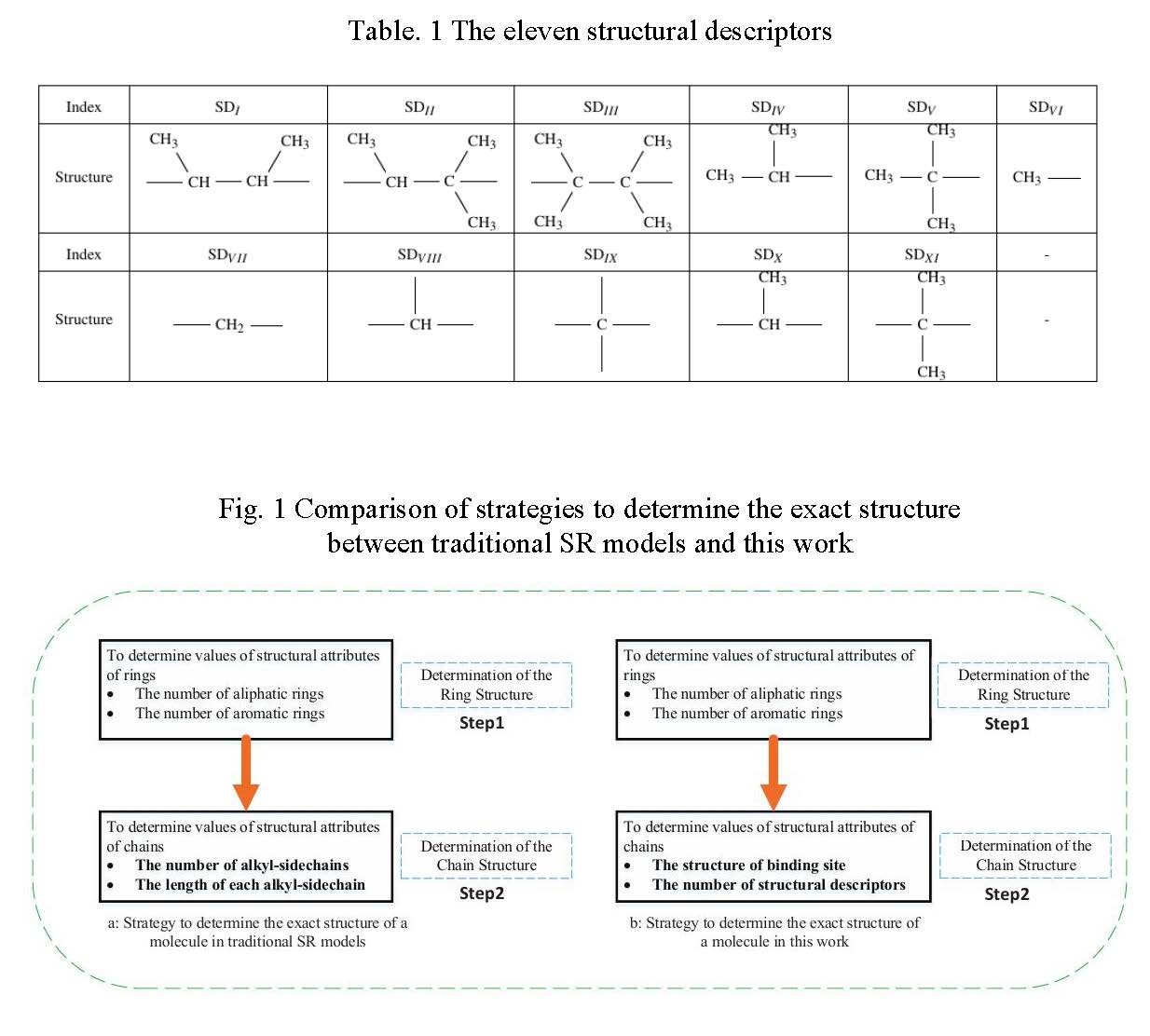
Eleven structural descriptors(SDs) are proposed as building increments of hydrocarbon chains, as shown in Table 1. SDI_to_IX are consistent with functional groups in group contribution method2. Therefore, contribution values on properties can be directly used for SDI_to_IX.SDX and SDXI are created to count the number of partial overlapping sections between SDI_to_V. Contribution values of SDX and SDXI can be derived from SDI_to_IX. The number of eleven structural descriptors form the structural vector to represent the exact structure of saturated acyclic hydrocarbon chains at the level of property estimation.
We designed a building diagram to apply the novel structural vector into stochastic reconstruction method. Fig. 1 shows the difference between traditional SR method and the SR method in this work. In traditional SR method, building diagram just focuses on configuring structural attributes of molecules, including the number of rings and side chains and the length of each side chain. Then according to the determined structural attributes, predefined molecules in corresponding categories are selected. While in this work, we present different building strategies. In the step of configuring rings, we also determine the number of rings, including aromatic rings and aliphatic rings. In configuration of structures of paraffin and side chains, if they are isomeric, we determine the number of eleven structural descriptors, instead of the length of chains. For ring-containing components, it is necessary to determine the structure of binding sites of side chains. The hierarchy strategy can present the uniqueness of molecular components at the level of property estimation and simultaneously reduce the complexity of building procedure in molecular reconstruction.
A structural library of rings is predefined in this work. Due to the limits of boiling point temperature and carbon number of components, the library includes cyclopentane, cyclohexane and benzene. There are six types of binding sites. For naphthenes, they are CHcyc-CH2, CHcyc-CH and CHcyc-C. For aromatics, they are aC-CH2, aC-CH and aC-C. CHcyc-CH3 and aC-CH3 are not included because they are regarded as normal side chains. It is necessary to determine the structure of binding sites to calculate the properties of full molecules. Heteroatom-containing hydrocarbons are not considered in this work because the naphtha samples used in this paper only contain few content of sulfur. In this work, it is assumed that each generated mixture contains 5000 molecules.
Histogram and gamma PDFs are used in sampling process. For determining the type of molecules and whether the chain is normal, histogram is reasonable. Gamma PDFs are used in determining the number of rings and number of eleven structural descriptors. Since the chemical analysis suggests that weight fractions follow a gamma distribution against boil point temperature in homologous series, we impose the gamma PDFs on weight fractions in homologous series against boiling point temperature in this work.
An objective function is proposed by minimizing the relative error of the bulk properties of naphtha samples between experiment and mixing rules. Properties used in this work includes basic properties, distillation curve and detailed weight fractions of homologous series. The average properties of the generated mixture are calculated by mixing rules.
The new SR model were tested with 50 naphtha samples. Properties of the generated mixture in each run are very close to experimental values. The average relative errors of basic properties and distillation curve are all under 3.8%. The relative errors of basic properties and distillation curve for 50 tests are all under 4.95%. The model also shows good performance to predict detailed weight fractions of homologous series. This work decoupled the indispensable relationship between models and predefined molecules and can contribute to the study of molecular reconstruction under the shortage of analytical information. With enough prior knowledge, analytic information can also be coupled with this model.
- Ren, Y., et al., Molecular reconstruction: Recent progress toward composition modeling of petroleum fractions. 2019. 357: p. 761-775.
- Hukkerikar, A.S., et al., Group-contribution + (GC +) based estimation of properties of pure components: Improved property estimation and uncertainty analysis. Fluid Phase Equilibria, 2012. 321: p. 25-43.
- Neurock, M., et al., Monte carlo simulation of complex reaction systems: molecular structure and reactivity in modelling heavy oils. Chemical Engineering Science, 1990. 45(8): p. 2083-2088.