2020 Virtual AIChE Annual Meeting
(445c) Convection-Driven Intravascular Bioartificial Pancreas (iBAP) Enhances Solute Delivery to Macroencapsulated Human Islets.
Authors
Materials and Methods:
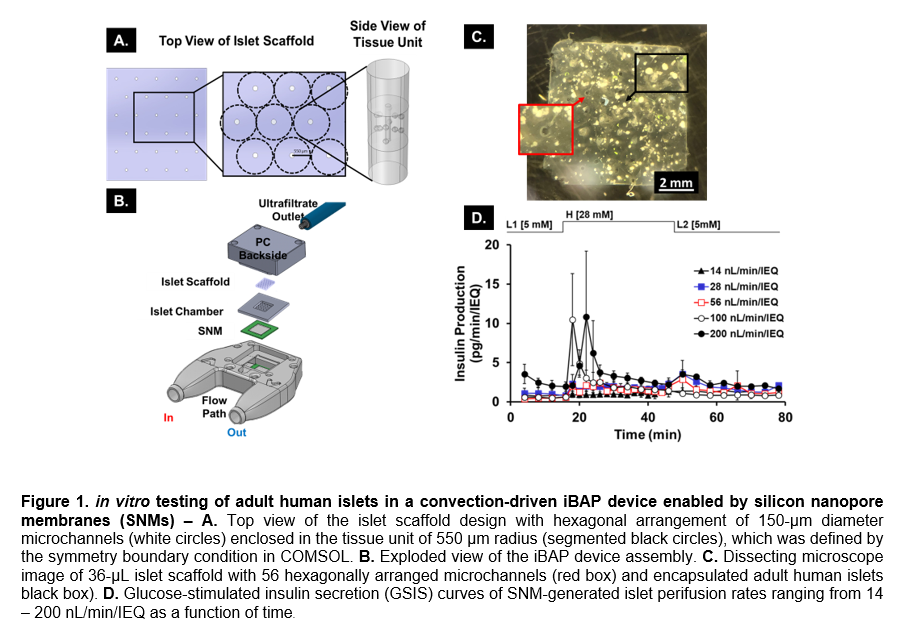
Oxygen modeling: The oxygen model used COMSOL Multiphysics to define our systemâs geometry (Figure 1.A.), which includes convective flow through the scaffoldâs microchannels and diffusive transport with reactive solute rates in the islet tissue. The post-processing cut-line feature in COMSOL was used to determine the worst-case oxygen profile scenario of each condition; i.e., the islets farthest away from the hexagonally arranged microchannels. The Navier-Stokes and mass continuity equations for incompressible Newtonian fluids were used to describe the velocity field due to convection, whereas consumption rates were assumed to follow Michaelis-Menten kinetics and dependent on the glucose concentration. This model established the oxygen consumption profiles of islet scaffolds at 14, 28, 56, 100 and 200 nL/min/IEQ.
In vitro set-up: The SNM-based iBAP (Figure 1.B.) was composed of a polycarbonate blood flow-path sandwiched by an SNM-islet chamber stack on each side. The CNC machined 316L stainless-steel islet chambers (Prototek Manufacturing Services, IncSunnyvale, CA)) held the adult human islets (UCSF Islet and Cellular Production Facility (San Francisco, CA) and Prodo Laboratories, Inc (Aliso Viejo, CA)) in a 3% (w/v) ultra-low gelling agarose scaffold (Sigma: 9012-36-6) patterned with microchannels (Figure.1.C.). Each islet chamber was sealed with a polycarbonate backside containing an ultrafiltrate (UF) outlet. A dynamic GSIS assay was performed by connecting the iBAP device to a mock circulatory flow system conditioned at 37°C and 5% CO2. The encased islets were stabilized in a 2-hour continuous flow period represented as the âpre-stimulatory phaseâ at 5 mM of glucose (L1). Ultrafiltrate samples were collected for the last 16 minutes of this period. The glucose concentration was then increased to 28 mM (H) and samples were collected for 30 minutes. Lastly, the glucose concentration was reduced to 5 mM (L2) and samples were collected for 32 minutes. The insulin content in the UF samples was quantified via an enzyme-linked immunosorbent assay (Mercodia:10-1113-01).
Results and Discussion: The oxygen model revealed that perifusion rates as low as 28 nL/min/IEQ provided sufficient oxygen to maintain insulin production, which requires oxygen tensions above the cut-off threshold value of 25 mmHg (or 0.034 mol/m3) to avoid inhibition of insulin secretion. In addition, GSIS curves of rates above 28 nL/min/IEQ exhibited the characteristic biphasic secretion pattern generally observed with human islets in traditional perifusion systems (Figure 1.D.) and included a shutdown of insulin production upon transition to low glucose (L2) medium. Therefore, the correlation between oxygen profile and GSIS kinetics for the perifusion rates of 14 and 28 nL/min/IEQ validate the oxygen model, given that 14 nL/min/IEQ exhibited poor glucose-insulin kinetics. Interestingly, the 100 nL/min/IEQ condition averaged a higher stimulation index (13.86±7.29) compared to the higher 200 nL/min/IEQ rate (5.30±2.48), suggesting that higher perifusion rates may affect insulin production possibly due to damage induced from shear forces at the periphery of the islets exposed to higher flows.
Conclusion: Human islets in the iBAP device matched the GSIS pattern observed with standard islet perifusion studies indicating the islet scaffold can ensure rapid glucose-insulin kinetics under convective mode. Furthermore, a clinically relevant insulin production was observed with a relatively small density of islets in vitro since the most promising perifusion rates of 100 and 200 nL/min/IEQ correlated to islet densities as little as 2.5% volume of IEQs (~500 IEQs) per volume of a 36-µL islet scaffold. Therefore, this work will inform future islet dosing experiments and device size necessary to systemically deliver insulin to treat T1D.
Acknowledgements: This research was supported by JDRF (Grant #: 3-SRA-015-37-Q-R) and the NIH/NIDDK Small Business Innovation Research (SBIR) Program (Project #: 5R44DK104299-03). would also like to thank colleagues Drs. Rachel Gurlin and Rebecca Gologorsky for their insightful contributions.