2020 Virtual AIChE Annual Meeting
(350g) Understanding and Predicting the Efficacy of Cold Flow Improvers
Authors
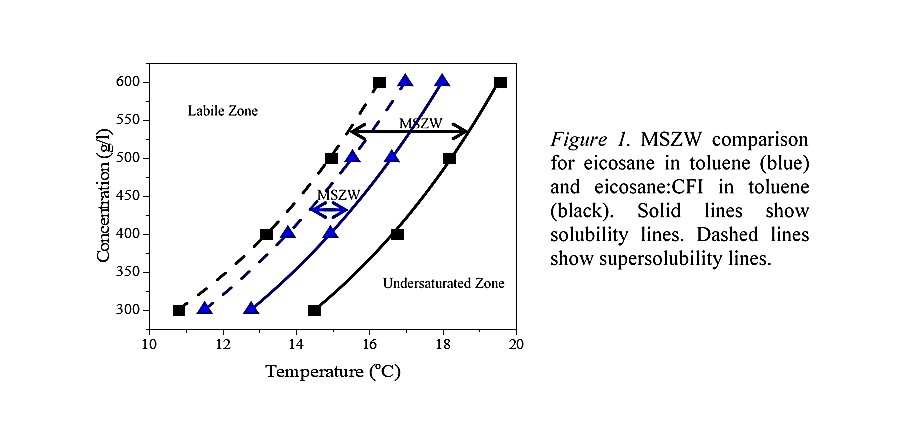
A polythermal screen of 5 CFIs was performed for the model system of eicosane in toluene solution, with the KBHR approach3,4 undertaken to assess nucleation kinetics. Eicosane in toluene was found to nucleate through instantaneous nucleation (IN), whereas all CFIs were found to alter the nucleation pathway to progressive nucleation (PN). This is important to product flow assurance, as IN would cause catastrophic wax nucleation throughout the product, whereas the capability to alter this to PN would mean crystals would not appear all at once and flow would still be possible. One CFI was found to have the largest efficacy, increasing the metastable zone width (MSZW) to nucleation by 3 times, shown in figure 1, and was used for further study.
For the IbD methodology a second miscible solvent was required, in order to perform antisolvent crystallization experiments. For this purpose, acetone was selected, owing to the low solubility of waxes in this solvent. The solubility in the absence and presence of the CFI over a large compositional range of toluene:acetone mixtures in 10vol% increments was assessed, with a sharp decrease found with increasing acetone content. IbD results were obtained following a workflow previously developed by the author 2. Induction times (the time to crystallization after a level of supersaturation is reached) were reduced by 4 orders of magnitude over the range of supersaturations studied, demonstrating the ability to accelerate nucleation. Furthermore, results were found to lie on one curve, meaning extrapolation of results obtained for short induction times could be used to determine those at longer induction times. These findings are extremely important for predictive testing as it enables experimental work to be conducted in a short time frame, but results obtained can be used to determine effects over longer timescales that would be present in industrial product applications and are not accessible in normal laboratory experimental work.
References
1) ATC - Technical Committee of Petroleum Additive Manufacturers in Europe, 2013, 68.
2) P. L. Kaskiewicz, G. Xu, X. Lai, N. J. Warren, K. J. Roberts, C. Morton, P. Dowding and N. George, Organic Process Research and Development, 2019, 23, 1948â1959.
3) D. Kashchiev, A. Borissova, R. B. Hammond and K. J. Roberts, Journal of Crystal Growth, 2010, 312, 698â704.
4) D. Kashchiev, A. Borissova, R. B. Hammond and K. J. Roberts, Journal of Physical Chemistry B, 2010, 114, 5441â5446.