2020 Virtual AIChE Annual Meeting
(346e) Influence of Oxygen Coverage on the Dominant Routes Towards Furanic Oxidation
Authors
Naveen Agrawal - Presenter, Pennsylvania State University
Lesli Mark, University of Colorado, Boulder
Alex Roman, University of Colorado Boulder
Adam Holewinski, University of Colorado
James Medlin, University of Colorado
Michael Janik, The Pennsylvania State University
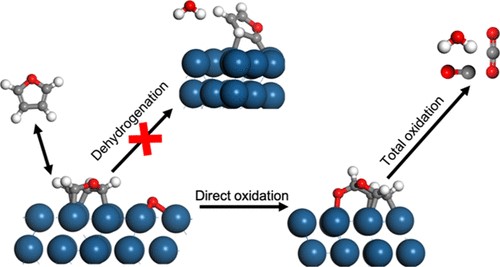
Electrochemical conversion of biomass derived furanics to industry relevant chemicals show strong promise in the area of renewable chemistry. But the understanding of the mechanism on Pt catalysts is lacking. Using a simple approach to calculation of reaction energy and potential dependent electrochemical activation barriers, it is now possible to compute the free energy landscape of complex furanic conversion pathways. We have earlier showed with Density Functional Theory (DFT) calculations that decarboxylation of furoic acid (C-C cleavage) limits the rate of furfural oxidation which seems to consistent with high selectivity towards furoic acid at moderate potentials (0.8V-1V-RHE)[1]. However modeling the reaction energetics at higher potentials is complicated with increasing coverage of oxygen on catalytic surface. Experiments show increasing selectivity towards oxidation products deeper than furoic acid at higher potentials on Pt (111). This is hypothesized to occur due to possibility of additional pathways to furanic ring oxidation facilitated through the increasing coverage of oxygen at higher potentials on the modelled clean Pt (111) surface. Energetics of decarboxylation at partial oxide layer of PtO (100) and other thermodynamically feasible oxide models do not explain the increased rate of decarboxylation at higher potentials. To investigate the effect of partial oxygen coverage for the reaction of furanics on Pt (111), temperature-programmed desorption (TPD), high-resolution electron energy loss spectroscopy (HREELS), and density functional theory (DFT) calculations were conducted. HREEL spectra and DFT calculations indicated that furan initially reacted through oxygen addition to the ring to produce surface-adsorbed furanone intermediates. HREELS and DFT calculations suggested that alcohol oxidation proceeded through dehydrogenation at the hydroxyl and methylene groups and subsequent oxidation to produce a carboxylate intermediate [2]. These results suggest that direct insertion of oxygen into the furanic ring is an energetically accessible pathway at different stages of oxidation. Insertion of oxygen/hydroxyl into furanic ring prior to C-C cleavage can allow access to reaction pathways explaining synthesis of hydroxyfuroic acid in furfural electrochemical oxidation on Pt [3] and might allow us to explain the shift in increased rate of furoic acid oxidation at higher potentials through oxygen insertion.
References:
- Gong, L., Agrawal, N., Roman, A., Holewinski, A., & Janik, M. J. (2019). Density functional theory study of furfural electrochemical oxidation on the Pt (1 1 1) surface. Journal of catalysis, 373, 322-335.
- Mark, L. O., Agrawal, N., Román, A. M., Holewinski, A., Janik, M. J., & Medlin, J. W. (2019). Insight into the oxidation mechanism of furanic compounds on Pt (111). ACS Catalysis, 9(12), 11360-11370.
- RomaÌn, A. M., Hasse, J. C., Medlin, J. W., & Holewinski, A. (2019). Elucidating Acidic Electro-Oxidation Pathways of Furfural on Platinum. ACS Catalysis, 9(11), 10305-10316.