2020 Virtual AIChE Annual Meeting
(219d) Meso-Kinetic Transient Optimization Via Machine Learning
Authors
Matthew Kunz - Presenter, Idaho National Laboratory
Rakesh Batchu, Idaho National Laboratory
Yixiao Wang, Idaho National Laboratory
Adam Yonge, Georgia Institute of Technology
Andrew Medford, Georgia Institute of Technology
Zongtang Fang, Idaho National Laboratory
Denis Constales, University of Ghent
Gregory Yablonsky, Washington University in St. Louis
Rebecca Fushimi, Idaho National Laboratory
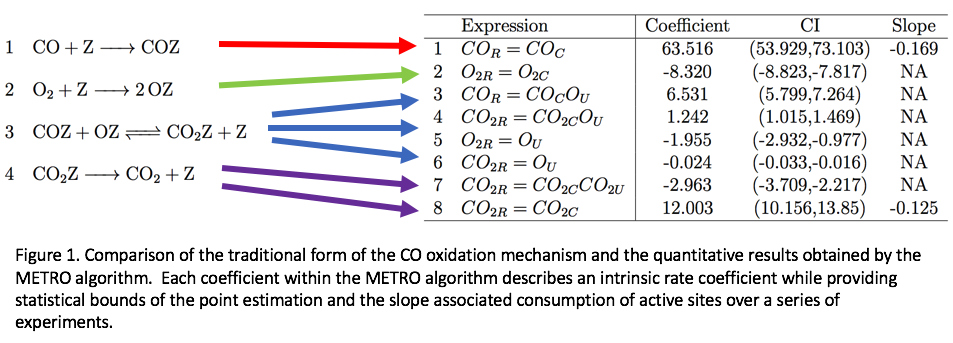
Understanding the set of elementary steps and kinetics in a given reaction is extremely valuable for making informed decisions about next-generation catalyst materials. Traditionally, this information has been collected through extensive physics-based simulation to construct first-principles models, and/or fitting kinetic models with or without underlying mechanisms to experimental data. However, due to the rough granularity of commonly used steady-state analysis techniques, it is common that multiple first-principle models fit the experimental results equally well. As such, it is often up to the domain experts to infer the kinetics based on chemical intuition. We propose to help bridge the gap between simulation and experiment through the combination of highly dense transient kinetic measurement and chemically-inspired machine learning techniques. Specifically, we leverage non-convex variable selection techniques within a framework constrained by a chemical mechanism to obtain a quasi-equilibrium expression of the reaction mechanism purely based on experimental data. This methodology, referred to as MEso-kinetic TRansient Optimization (METRO), is validated against simulated responses where true kinetic coefficients are known, and is shown to accurately estimate these parameters without any a priori information. Moreover, the method is applied to experimental data from well explored reactions such as carbon monoxide oxidation and ammonia decomposition.The METRO methodology is proposed as a compliment to bottom-up microkinetic modeling, where the top-down fitting can provide insight into the reaction mechanism and extract intrinsic kinetic parameters of elementary processes directly from experimental transient kinetic data.