2020 Virtual AIChE Annual Meeting
(19e) Selective Reduction of Carboxylic Acids to Aldehydes over Promoted MoO3 Catalyst
Authors
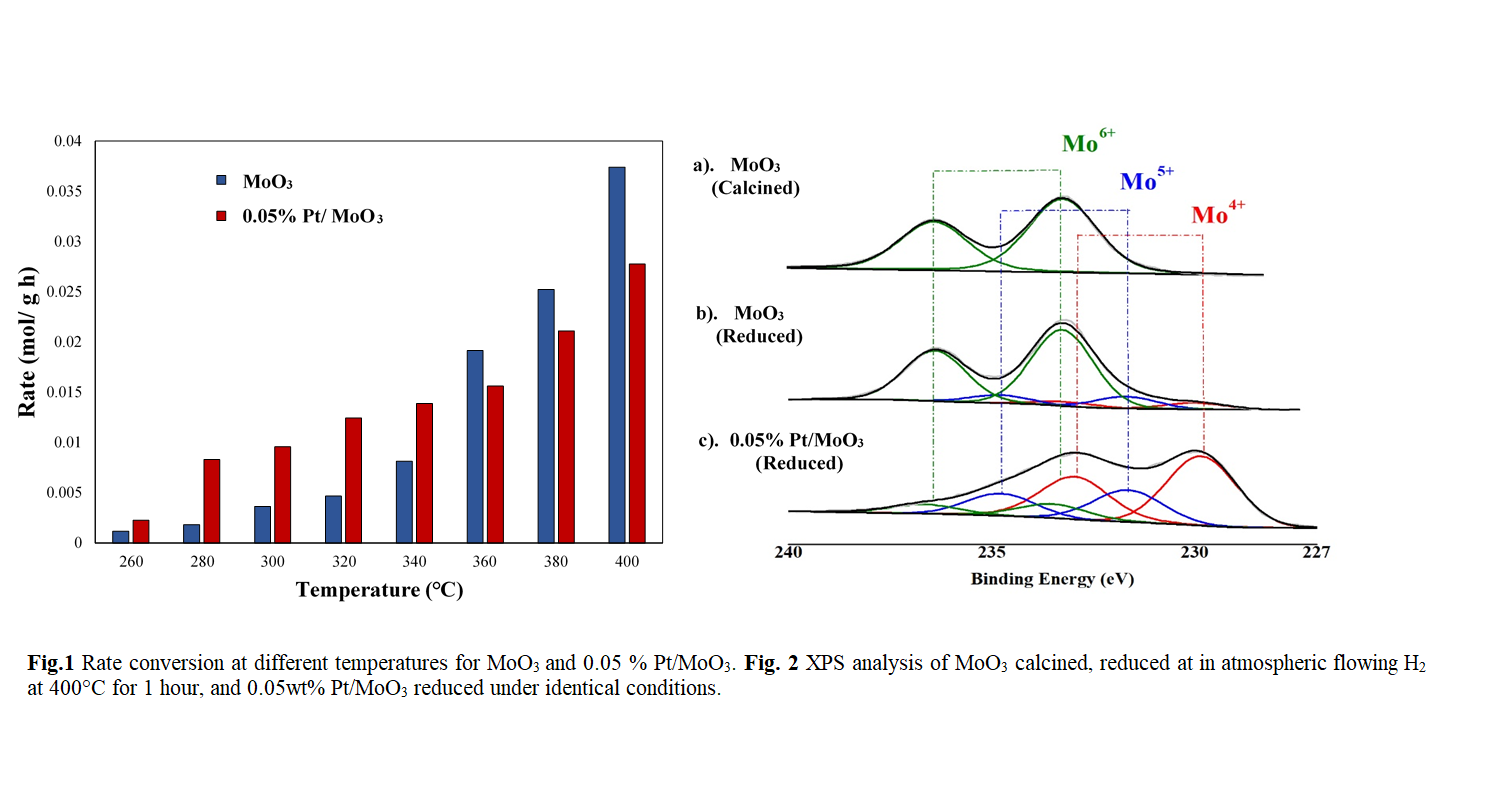
In this contribution, we report a new chemistry that has been explored with aldehydes and ketones, reverse mars van Krevelen chemistry over MoOx, for the selective conversion of carboxylic acids. Our study focusses on the selective conversion of pentanoic acid (PA) to aldehyde over MoO3 at different temperatures via hydrodeoxygenation (HDO) reaction. Here, we hypothesize that the selective C-O cleavage reactions are occurring over the defects on the sub stoichiometric oxide via reverse Mar-Vars Krevelen chemistry. Reducibility of MoO3 is well known to be challenging at low temperatures, therefore in order to facilitate the reduction process, very low loadings of platinum (Pt) were incorporated on the catalyst (0.05%Pt/MoO3). The net effect is the increase on the rate of conversion of PA at lower temperature over MoO3 by hydrogen spillover to promote partial reduction of the MoOx while minimizing side reactions that may occur on the metal directly. X-Ray photoelectron spectroscopy (XPS) was used to investigate effect of H2 during the partial reduction on both MoO3 and 0.05% Pt/MoO3 catalysts. Kinetics analysis reveals that low loading of Pt on MoO3 decrease the apparent activation barrier for acid conversion by over 33 kJ/mol.