2020 Virtual AIChE Annual Meeting
(152a) Detection of Atrazine and Its Metabolites Using Photonic Molecularly Imprinted Polymers
Authors
We developed an inexpensive label-free photonic sensor for the rapid detection of atrazine and its metabolites for field use. The sensor is composed of a molecularly imprinted polymeric (MIP) porous film that selectively captures atrazine molecules; the film pore structure is an inverse opal and its optical properties change when atrazine is captured.
Molecularly Imprinted Polymers (MIPs) have highly specific adsorption sites in their polymeric structure which leads them to selectively adsorb target molecules in those sites. Using a colloidal crystal of silica nanoparticles, a porous structure has been created in the polymer to facilitate target molecules movements through the thin film of polymer. By combining colloidal-crystal templating and molecular imprinting technique, a pH-paper-like sensor for a label-free colorimetric detection of atrazine and its metabolites in aqueous solutions and wetlands is fabricated. The void spaces of pores with different sizes and morphology generates Bragg diffraction and the wavelength of the peak changes when rebinding of target molecules happens. An optical signal via a change in Bragg diffraction is produced to detect atrazine. This optical property gives us an appropriate method of transducing recognition of target to a sensible signal.
Silica particle colloidal crystals were obtained by vertical self-assembly from 0.1% volume fraction suspensions in ethanol at 50°C. MIPs were fabricated with acrylic acid as the functional monomer, ethylene glycol dimethacrylate as crosslinker and UV-polymerization. The films, approximately 5 mm thin, were supported on polymethylmethacrylate (PMMA) slides.
Acrylic acid (AA), ethyleneglycol dimethacrylate (EGDMA), and azobisisobutyronitrile (AIBN) have been used as monomer, crosslinker, and initiator in polymerization precursor, respectively. Target molecules were also added to polymerization precure at 1:80 molar ratio to monomers. Then, a PMMA plastic slides on each sides of glass slides with depositions were firmly held together while immersed in polymerization precursor from one end. The polymerization precursor infiltrated through sandwich structure. Then, sandwich structure was held before UV light (365 nm) for 3 hours for the polymerization to take place. Inverse opal was created within polymer film by soaking PMMA slides in hydrofluoric acid (5%) and the silica particles were etched away, leaving empty cavities in the polymer. Finally, the target molecules were removed from film by washing with acetic acid and ethanol with ratio of 1:9. The MIPs were washed 3 times, for 15 minutes each time, using a shaker to enhance efficiency. Since detecting the metabolites is as essential as atrazine itself, MIPs were fabricated to detect DEA and DIA following the same steps.
Colloidal crystals were imaged by a FEI Quanta 600 FEG scanning electron microscopy to characterize the morphology of the crystal structure and the number of layers.
UV-Vis spectrophotometry was used to measure film surface reflectance to verify any swelling or shrinkage within the hydrogel. The reflectance spectra were recorded before and after exposure to clean water or atrazine over a wavelength range of 200â800 nm, using a benchtop double-beam UVâvisible spectrophotometer equipped with a specular reflection accessory at a fixed angle of 30°.
Kinetic tests were conducted to investigate the time required for MIPs to reach equilibrium with a solution. Reflectance spectra were measured in specific time windows until the peak wavelength did not show any further changes. In kinetics test, the MIPs took 20 minutes to reach equilibrium, meaning it is necessary to incubate MIPs in unknown solutions for 20 minutes for the precise quantification of atrazine concentration.
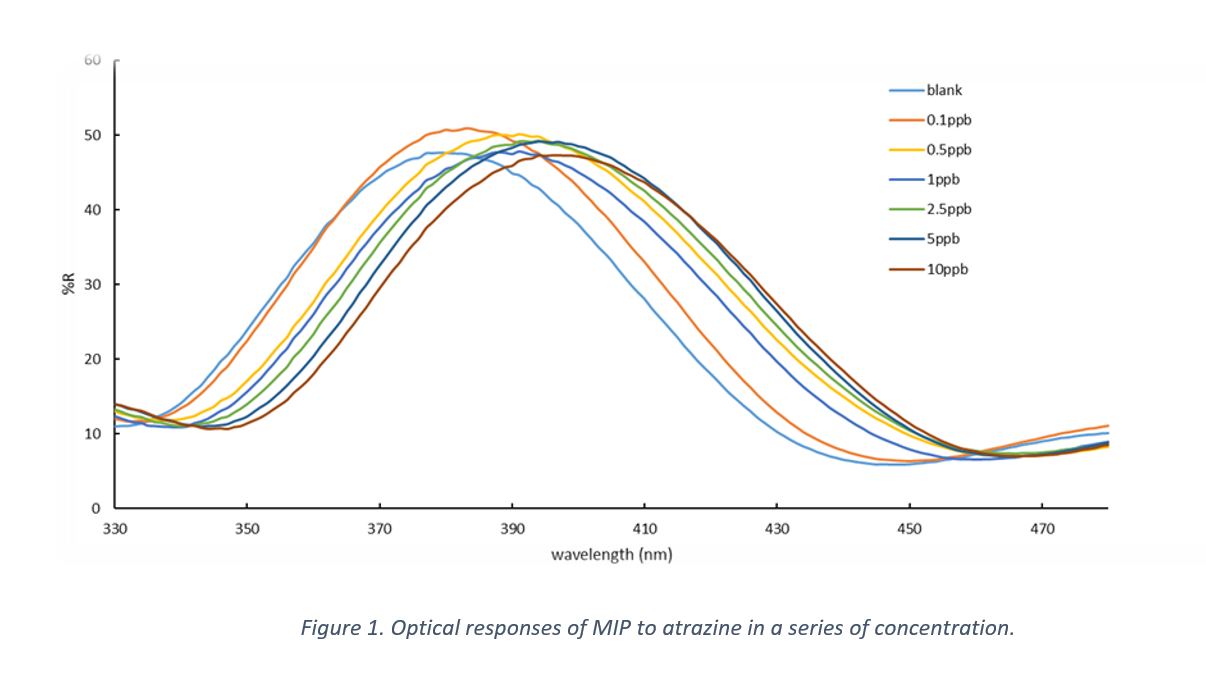
The reflectance spectra of MIPs incubated in different solutions are illustrated in Figure 1. Changes in peak wavelength are a sign of swelling or shrinkage in hydrogel; in other words, adsorption of target molecules in binding sites of the polymer. The immersion of MIPs in solutions with higher concentration of atrazine changed the peak wavelength of the reflectance spectrum, as shown in Figure 1. In each step, peak wavelength increases in comparison with last more diluted solution which is a result of rebinding of target molecules in void binding sites in the structure of hydrogel. This target rebinding forms changes in porous structure of film, yielding different responses in reflectance spectrum each step. Then, based on this behavior and saturation time (20 minutes), MIPs were incubated in standard solutions with specific concentrations for 20 minutes with a gentle shaking and their reflectance were measure each step. The standard solutions, validated by LCMS, were used for measuring peak wavelength shifts for MIPs and to generate a calibration curve for quantifying unknown solution concentration of atrazine based on changes in wavelength shift.
The protocol for calibrating metabolites MIPs has been the same. Standard solutions validated by LCMS have been prepared and used to generate calibration curves by incubating metabolites MIPs in those standard solutions for 20 minutes on a shaker.
The fabricated sensor constitutes a simple, fast, and efficient tools to detect atrazine and metabolites in wetlands and also, quantify their concentration in order to make sure it does not go far higher than MCL. The thin film MIP-based sensors have a dynamic range of 0.14-12.3 ppb and calibration curves have been generated incubating MIPs in standard solutions with different concentrations.