2020 Virtual AIChE Annual Meeting
(118h) Multiple Particle Tracking Detects Changes in Brain Extracellular Matrix Structure
Authors
Approach: Our approach uses multiple particle tracking (MPT), a technique that leverages fluorescent microscopy to capture the motion of nanoparticles in real-time. MPT is unique in that the microscopic behavior of hundreds to thousands of individual particles can be tracked simultaneously, while retaining single particle resolution. The motions exhibited by particles provide information about the environment in which they reside, and the ability to track the movement of individual particles provides high spatial resolution. By using sub 60 nm tracer particles with polyethylene glycol (PEG) surface coating, we are able to capture diffusion within the narrow channels of the brain extracellular space (ECS) and evade adhesive interactions with cellular components. The motion of these particles is thus influenced by local fluid properties of the ECS and structural properties of the local ECM. To determine whether MPT was sensitive enough to detect changes in local ECM structure, we applied it to two separate scenarios ex vivo. First, we enzymatically-induced changes in ECM structure in rat brain slices by treating them with one of two ECM degrading enzymes. We then tested whether MPT could detect a rearrangement of brain ECM that occurs naturally during neurodevelopment. For this, MPT experiments were performed in brain slices taken from rats spanning a range of ages within the critical period of neurodevelopment.
Materials and Methods: Acute whole hemisphere rat brain slices were prepared by taking 300 µm-thick coronal sections from freshly euthanized Sprague-Dawley rats. Brain slices were cultured on membrane inserts at physiologically relevant conditions. Ex vivo experiments were split into two groups: (1) a group that applied exogenous enzymes to induce changes in brain ECM structure, and (2) a group that relied on a rearrangement of brain ECM that occurs naturally during development. For group (1), litter matched brain slices were collected from rats on postnatal days 35, 36, 37, and 38 (P35-P38). Within 24 h of euthanasia, slices were treated with either chondroitinase ABC (ChABC, 0.4 U/mL), hyaluronidase (HYase, 35 U/mL), or culture media. Treatments lasted for 2 h, at which point MPT was performed. 30 min prior to video acquisition, brain slices were injected with 51 nm PEG-coated fluorescent polystyrene nanoparticles (PS-PEG). Videos of nanoparticle displacements were taken from the cortex of each slice at 33 frames-per-second and 100x magnification for 651 frames using a cMOS camera mounted on a confocal microscope. Nanoparticle trajectories, trajectory mean squared displacements (MSDs), and effective diffusion coefficients (Deff) were calculated using a Python package developed within our group. For group (2), slices were collected from P14, P21, P28, and P35 rats. Within 24 h of slice collection, the same 51 nm PS-PEG nanoparticles were injected into brain slices and MPT was carried out in the cortex. Videos were collected and results generated using the same methodology. In both instances, a change in the presence of PNNs was used as an indicator of changes in brain ECM structure. To detect changes in the presence of PNNs, brain slices were stained with fluorescein-labeled wisteria floribunda agglutinin (WFA) lectin at 10 µg/mL for 12 h at 4oC and imaged using a confocal microscope.
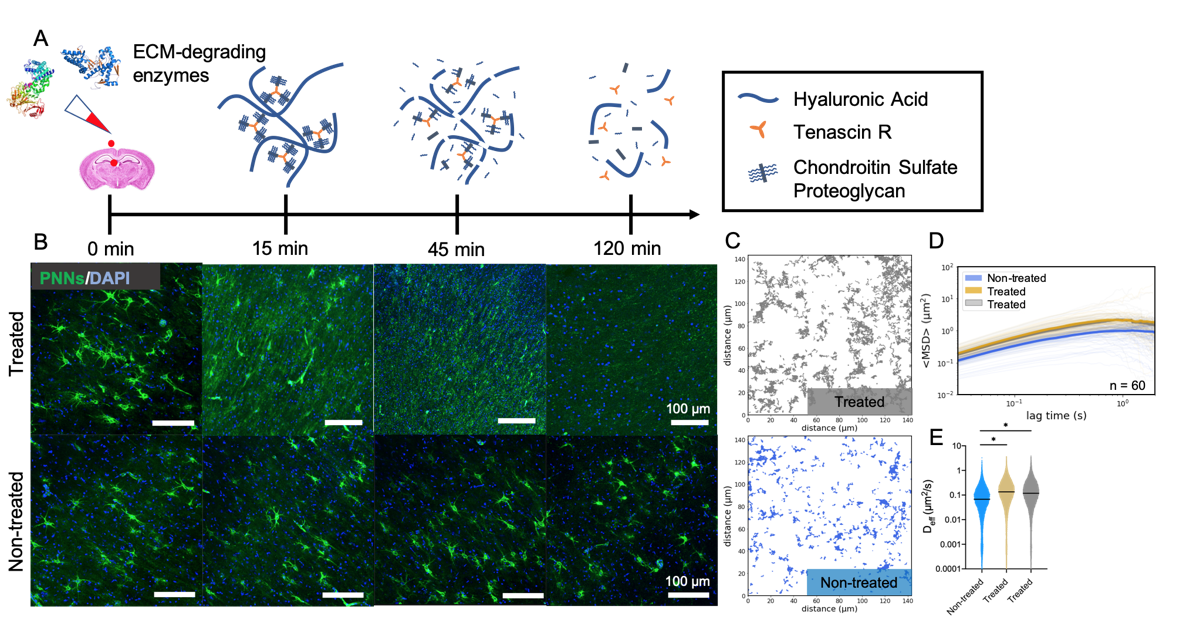
Results: Both treatments, ChABC and HYase, led to a complete loss of PNNs in P35 rat brain slices within 2 h (Figure 1). PNNs present in slices treated with culture media were not affected. Nanoparticles explored a greater area, moved faster, and had increased diffusivities when diffusing in enzyme-treated brain tissue. Despite having the same number of total trajectories (1478 and 1732 for non-treated and treated, respectively), nanoparticles surveyed a greater fraction of the ECS when diffusing in the slice treated with an ECM-degrading enzyme. In enzyme-treated slices, geometrically averaged mean-squared displacements (<MSD>) of nanoparticle trajectories were greater in magnitude for all lag times between 0 and 2 s. Lastly, nanoparticles diffusing in treated slices had significantly larger Deff values than those diffusing in non-treated slices. We then transitioned to the age dependent study. PNNs stained using WFA did not appear qualitatively until 21 days after birth (P21), and preferentially formed around parvalbumin-expressing (PVA+) interneurons. The areal density of PNNs in the cortex increased throughout development, with significant differences existing between P14 and P28, P14 and P35, and P21 and P35 (p < 0.05) groups. Tracking revealed an inverse relationship between nanoparticle diffusive ability and brain age. <MSD> profiles decrease in magnitude as pup age increases from 14 days to 21, 28 and 35 days after birth. Distributions of Deff values at a 0.33 s lag time shift to lower values as the brain develops and correspondingly PNN density increases.
Conclusions: Brain ECM plays critical roles throughout neurodevelopment, helps preserve long-term neuronal health, and facilitates repair in response to injury. Altered ECM structure is thought to be involved in the pathophysiology of many neurological diseases, and the implications of altered PNN integrity on neuronal plasticity and activity has garnered significant attention in recent decades. Until now, probing real-time changes in ECM structure, especially changes occurring at the cellular level in living tissue, has been challenging. Our findings suggest that MPT is capable of this. In addition to leveraging the brainâs natural tendency to restructure during the critical period of development, we also applied MPT to ex vivo hemispheric brain slices undergoing an enzymatically induced breakdown of ECM. In both instances, significant differences in nanoparticle diffusive ability existed between experimental groups. The further application of MPT in studying ECM structure could more explicitly define mechanisms involved in neurological disease progression and open new avenues of therapeutic intervention. Additionally, MPT can enhance our baseline understanding of the structure-function relationships of the brain under normal physiological conditions and has the potential to become used as one marker of neurological disease severity.
Figure 1. MPT detects enzymatically-induced breakdown of PNNs in rat brain slices ex vivo. (A) Schematic representation of PNN breakdown following treatment with HYase or ChABC. (B) Representative 20x magnification images taken from the cortex of P35 rat brain slices either treated with an ECM-degrading enzyme or not treated. Rows represent treatment group. Columns represent treatment time. (C) Representative trajectory maps generated from MPT experiments carried out in non-treated (blue) and HYase-treated (grey) P35 brain slices ex vivo. (D) Geometrically ensemble averaged <MSD> versus lag time quantified from trajectories of nanoparticles diffusing in non-treated (blue), ChABC-treated (gold), and HYase-treated (grey) P35 brain slices. Faint lines represent individual videos (60 total per group). Bold line represents the mean of 60 videos. (E) Scatter plots of Deff values generated from all videos (n = 5) in all slices (n = 3) in all brains (n = 4), separated by treatment group. Bars show median value (95% CI). * denotes significant differences (Kruskal-Wallis test) between groups, after adjusting for multiple comparisons. (p < 0.05)