(700e) Engineering of Spherical Lactose and D-Mannitol: Improving Excipient Particles for the Optimized Manufacture of Pharmaceutical Solid Dosage Forms
AIChE Annual Meeting
2022
2022 Annual Meeting
Pharmaceutical Discovery, Development and Manufacturing Forum
Advancements in Particle Engineering and Material Sciences in Pharmaceutical Process Development II
Friday, November 18, 2022 - 9:24am to 9:45am
Saccharides are naturally occurring compounds widely used in the pharmaceutical industry as excipients. Excipients are added to the formulation alongside the active pharmaceutical ingredient (API) to improve its processability, bioavailability, and stability. Lactose (LAC), a disaccharide, and D-mannitol (MAN), a polyol, are two widely used saccharides in pharmaceutical formulations. These are commonly used as fillers/diluents in solid dosage forms, such as tablets, pellets, capsules, dry powder inhalers (DPIs), etc., and have a critical role in guaranteeing the adequate performance of the API from manufacturing to its delivery to patients. However, to ensure that these excipients adequately fulfill their role in assuring that different dosage forms present the necessary quality attributes, their particle properties have, many times, to be optimized. Spherical particles, compared to more irregular-shaped ones, have some advantages, particularly in terms of processability. Their improved flow can significantly ease the manufacture of solid dosage forms and sometimes even beneficially impact delivery (i.e., DPIs). Likewise, in this work, we compare the ability of different engineering techniques to obtain stable spherical particles of LAC and MAN that could optimize manufacture and yield improved solid dosage forms.
Materials and Methods
Spray-drying and spray-congealing
Spray-drying and spray-congealing were performed using a 4-M8-TriX spray dryer/chiller (ProCepT, Belgium). For spray-drying, the solutions were fed at a low flow rate (1.0 ─ 2.0 g/min) into a bi-fluid nozzle (Ø 1.2 mm) at a 0.1 ─ 0.5 bar pressure. The jet was dispersed into a 0.05 m3 drying tower working at a drying airflow rate of 0.2 ─ 0.3 m3/min, and the temperatures were varied between 180.0°C and 100.0°C. The particles were collected at the outlet between 30.0 and 60.0°C, depending on the inlet temperature and airflow rate. For spray-congealing, the materials were first placed in an oven, and once a melt was obtained, this was transferred to a heated vessel kept at 200.0°C. The molten fluid was pumped at 20.0 g/min from the vessel to a heated bi-fluid nozzle (Ø 1.2 mm) and atomized (0.44 bar) into a glass tower chilled at -10.0°C, using a nitrogen flow of 0.60 m3/min. The solidified particles were collected in a glass vessel at 13.0°C.
Inkjet printing
Inkjet printing was performed using the R&D inkjet system PixDro LP50 (Meyer Burger, Netherlands) at a constant spotting frequency of 1000 Hz. The substrate (PTFE K400) temperature was kept at 50 °C during printing. To obtain particles, ten droplets were ejected onto the same spot and subsequently left to dry for 1 s. This was repeated 100 times, amounting to 100 particles with a dispensed volume of approximately 1000 pL per particle.
Wide-angle X-ray scattering (WAXS) and FT-RAMAN
For WAXS, the powders were filled into 2 mm glass capillaries, and the measurements were performed in the angular range of 17 and 27° 2θ during 600 s at 30 counts/s. For this, monochromatic CuKα radiation (λ = 1.54 Å) in a combined laboratory small and wide-angle camera (S3-MICRO, Hocus X ray Systems GmbH, Austria) was used. The FT-RAMAN measurements were performed in a Raman Station™ 400F (Perkin Elmer, USA) using three exposures during 10 s in seven analysis spots, at a 2 cm-1 between 200 ─ 3278 cm-1.
Scanning electron microscopy (SEM)
The particles prepared were carefully placed on the pin mount specimen holder of the SEM equipment (Zeiss Ultra 55, Zeiss, Germany). Next, the samples were sputtered with platinum and carbon or gold-palladium and were visualized using a detector operated at an acceleration voltage of 5 kV or 22 kV.
Aerodynamic performance
The aerodynamic performance was evaluated via shot weight consistency and next generation impactor (NGI) studies. The shot weight consistency was assessed by shooting the inhalers ten times into a DUSA (Dosage Unit Sampling Apparatus). High-performance liquid chromatography (HPLC) was used to quantify the quantity of surrogate API released. Depending on the inhaler used, the NGI experiments were carried out at 90 or 60 L/min, and the flow was applied for 2 or 4 s, respectively, to ensure that 4 L of air were drawn via the mouthpiece over the inhaler. The API content in each part of the impactor was quantified via HPLC.
Dissolution performance
The dissolution was investigated using a 24- well plate (Greiner Bio-One™ 24-Well-Platten, Germany), where 2 mL of PBS (pH 7.4) preheated at 37°C were added to each well. The well-plate was placed in an incubator shaker adjusted to 250 pm and 37 °C. UV-Vis was used to quantify the surrogate API release after 1, 5, 10, 15, 30, and 60 min.
Results and Discussion
Engineering of LAC and MAN particles
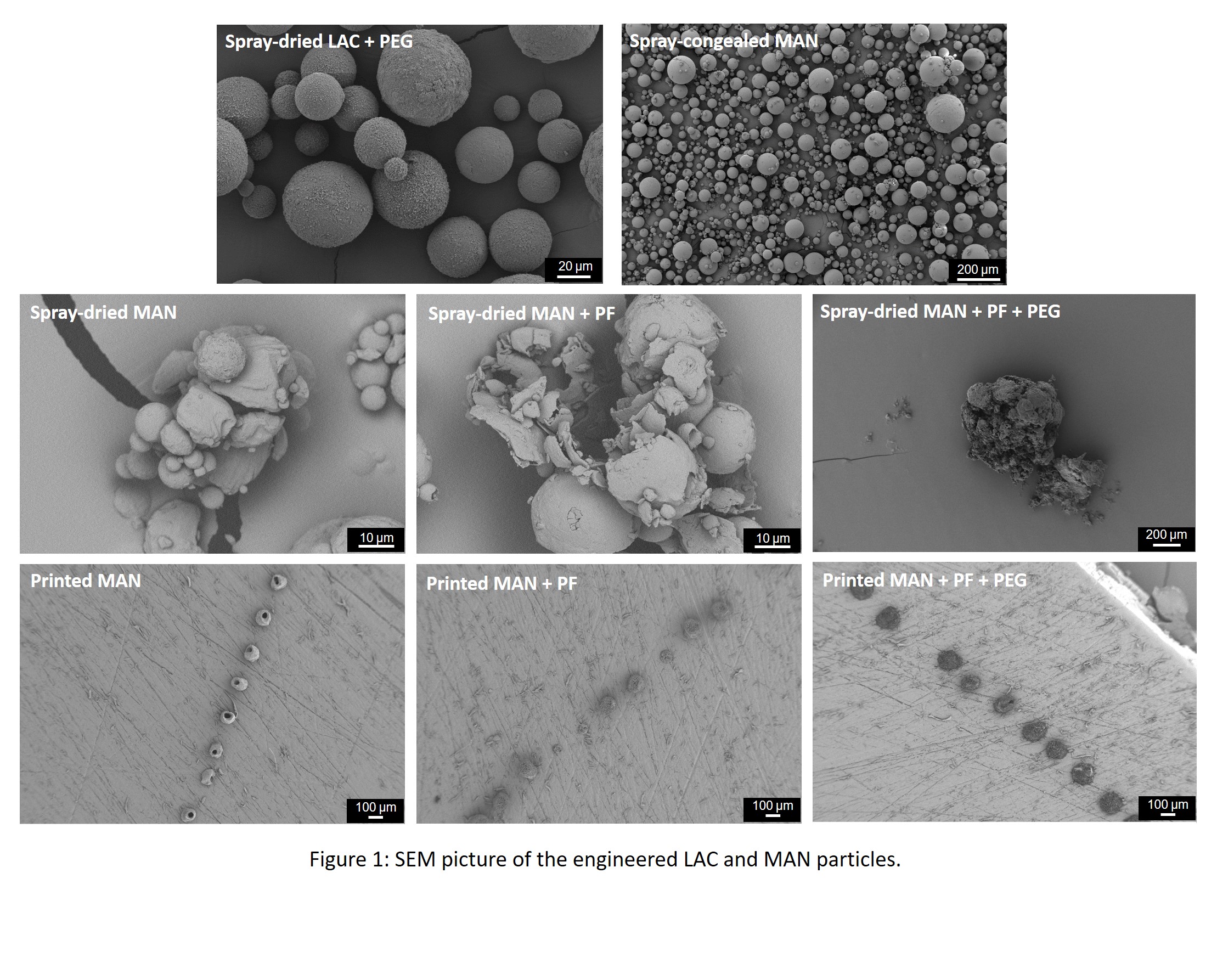
Crystalline particles are preferred over amorphous ones, as the latter have limited stability. Likewise, some of the spray-dried formulations of the sugars were processed in combination with poly(ethylene glycol) 200 (PEG) as this is a known crystal inducer. This was particularly important when engineering LAC, as this is known to be amorphous after spray-drying. MAN was spray-dried alone in the presence of the pore former (PF), ammonium bicarbonate, and PF combined with PEG. Moreover, because MAN does not degrade upon melting and has a less complex polymorphism, this was chosen to be further engineered via spray-congealing and inkjet printing.
Solid-state of the Engineered Particles
The LAC engineered in the presence of PEG showed to be crystalline and a mixture of the α- and β-polymorphs. In turn, MAN spray-dried alone yielded crystalline particles predominantly of the β-polymorph with traces of the α-form. The addition of PF alone and PF + PEG resulted in crystalline particles of MAN composed mainly of the β- and α-polymorphs, respectively. Printing the same MAN formulations resulted in a similar polymorphism for MAN alone and MAN + PF + PEG formulations. However, after printing, the formulations of MAN + PF, although crystalline, were constituted mainly by the α-polymorph. Spray-congealing of MAN resulted in crystalline particles predominately composed of the α-polymorph.
Morphology of the Engineered Particles
All the produced particles were spherical except the spray-dried MAN + PF + PEG, where large chunks of powder were obtained instead of discrete particles (Figure 1). The LAC + PEG particles presented a mean particle size of 60 µm, were monodispersed, and showed a rough needle-like surface. A similar surface structure was also found after printing when the MAN + PF + PEG formulation was processed. The MAN spray-dried alone was constituted of spherical, smooth agglomerated particles. The addition of PF to the formulation of MAN resulted in the production of shattered particles during spray-drying.
In contrast, discreet solid particles with pores were formed for inkjet printing. Moreover, it was observed that all the printed MAN particles had about 100 µm mean particle size and were very monodispersed. In contrast, the spray-dried MAN particles were much smaller and very polydispersed. Spray-congealing resulted in smooth polydispersed MAN particles with about 100 µm mean particle size.
Performance of the engineered particles
The spherical particles of LAC + PEG were compared with the classical tomahawk LAC monohydrate concerning their potential as DPI carriers. However, when the LAC + PEG particles were tested in a capsule-based device, these did not perform better than their tomahawk counterparts. Compared to other carrier particles, the spray-congealed MAN showed superior performance when used in binary blends with the API in combination with a reservoir-based device. For dissolution, we compared the printed particles of MAN with the spray-dried ones. Generally, a similar dissolution behavior was observed between printed and spray-dried particles, except for the formulations containing MAN + PEG + PF, where a more rapid dissolution was observed for the printed particles.
Conclusions
Our work showed that it is possible to spray-dry crystalline, monodispersed, spherical particles of LAC with a size suitable for use in DPI when adequately engineering the formulation and process parameters. However, if the compound does not degrade upon melting, spray-congealing might be a more suitable technique to manufacture superior inhalation carriers, as shown for MAN. Engineering of MAN via inkjet printing showed to be a potential alternative to spray-drying when the aim is to yield larger, monodisperse particles of saccharide with optimized dissolution behavior.
References
Alwossabi, E.S. Elamin, E.M.M. Ahmed, M. Abdelrahman, J. Medicinal Aromat. Plants 10 (7), 397 (2021). DOI: 10.35248/2167-0412.21.10.397
J.T. Pinto, S. Zellnitz, T.Guidi, F. Schiaretti, H. Schroettner and A. Paudel, Pharm. Res. 38, 1107-1123 (2021). DOI: 10.1007/s11095-021-03061-5
J.T. Pinto, S. Zellnitz, T. Guidi, E. Roblegg and A. Paudel, Mol. Pharm. 528 (7), 2827-2839 (2018). DOI: 10.1021/acs.molpharmaceut.8b00333