(635a) First European Demonstration Plant for the Synthesis of Renewable Ome Fuels
AIChE Annual Meeting
2022
2022 Annual Meeting
Fuels and Petrochemicals Division
Developments in Alternative Fuels and Enabling Technologies
Thursday, November 17, 2022 - 12:30pm to 12:47pm
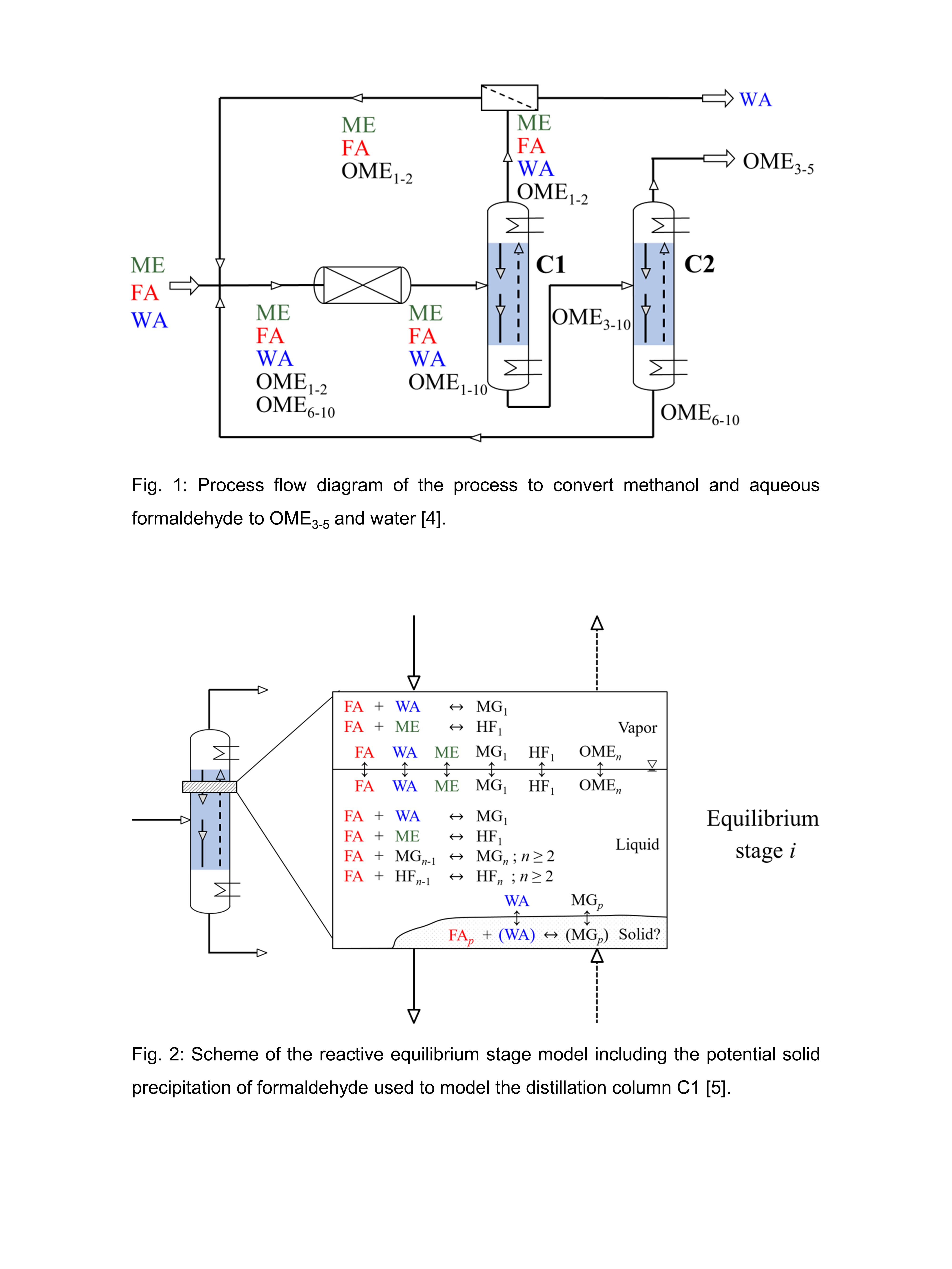
The most challenging unit of the process is the reactive distillation column C1 because of the high number of components present and because formaldehyde reacts uncatalyzed with methanol and water. Further, formaldehyde may easily precipitate since its solubility in these mixtures is limited. An equilibrium stage model for the reactive column C1 was developed, which is illustrated in Figure 2. This model includes a novel solid-liquid equilibrium model for formaldehyde conceived by the University of Kaiserslautern [5]. For the first time, the column is verified experimentally at process-relevant conditions including high concentrations of formaldehyde in the feed (> 0.10 g/g).
A further challenge is the separation of water from mixtures containing formaldehyde, methanol and OME. Because of the presence of multiple azeotropes, distillation is not the preferred option for this task [4]. Therefore, our plant uses a membrane unit, developed by the German company DBI Gas- und Umwelttechnik GmbH, that contains an innovative inorganic membrane material to selectively remove water from formaldehyde-containing mixtures as it was also but on a smaller scale investigated by Schmitz et al. [6].
In this contribution, we introduce the demonstration plant for OME synthesis at the Campus Straubing of the Technical University of Munich. The main challenges regarding the operation of the different units are discussed. We present the results of long-term steady-state experiments of the plant running in closed loop. In detail, we are looking at the performances of the reactor, the distillation column C1, and the membrane unit. Experimental profiles are presented and compared to process simulations. The present work shows that the individual as well as the combined operation of all process units is feasible despite the existing challenges and presents a relevant step on the way to a non-fossil transportation era.
References
[1] M. Härtl, K. Gaukel, D. Pélerin, G. Wachtmeister. MTZ Worldw. 78 (2) (2017) 52-29. DOI: 10.1007/s38313-016-0163-6
[2] M. Münz, A. Mokros, D. Töpfer, C. Beidl. MTZ Worldw. 79 (3) (2018) 16-21. DOI: 10.1007/s38313-017-0185-8
[3] A. Ferre, J. Voggenreiter, Y. Tönges, J. Burger. MTZ Worldw. 82 (5) (2020) 26-42. DOI: 10.1007/s38313-021-0639-x
[4] N. Schmitz, E. Ströfer, J. Burger, H. Hasse. Ind. Eng. Chem. Res. 56 (2017) 11519-11530. DOI: 10.1021/acs.iecr.7b02314
[5] C. F. Breitkreuz, J. Burger, H. Hasse, Ind. Eng. Chem. Res. 61 (2022) 1871-1884. DOI: 10.1021/acs.iecr.1c04275
[6] N. Schmitz, C. F. Breitkreuz, E. Ströfer, J. Burger, H. Hasse. J. Membr. Sci. 564 (2018) 806-812. DOI: 10.1016/j.memsci.2018.07.053