(563f) Award Submission: Next-Generation Tattoo-Ink for Improved Endoscopic Imaging
AIChE Annual Meeting
2022
2022 Annual Meeting
Nanoscale Science and Engineering Forum
Nanotechnology approaches to diagnostics, implants, templating and assembly
Wednesday, November 16, 2022 - 5:35pm to 6:00pm
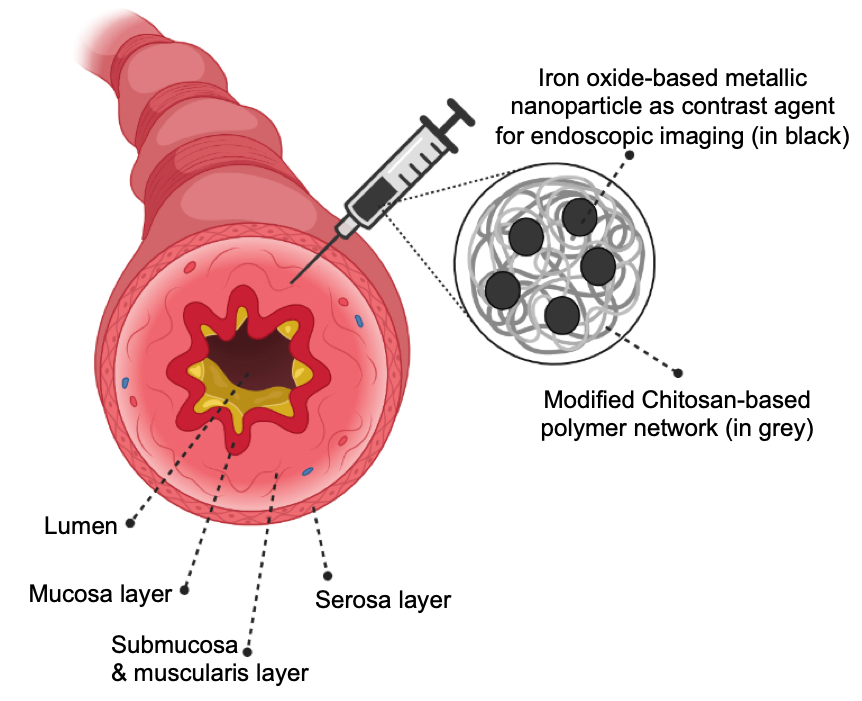
Polysaccharide-coated iron oxide nanoparticles were synthesized using a one-pot co-precipitation method. A library of cationic biomaterial derivatives was prepared based on variable molecular weight, terminal functional group, and chemical functionalization including thiolation, quaternization. All biomaterial derivatives were characterized using FTIR spectroscopy; amine and thiol contents were measured using ninhydrin and elman’s assay, respectively. A series of biomaterial-based tattoo-ink candidates were generated by modulating the ratio between the contrast agent and the biomaterial-based coating. Our tattoo-ink formulations, 0.25-2μm in hydrodynamic diameter, demonstrated near-equivalent contrast ex vivo with fresh porcine intestinal tissue as that shown by commercial inks, while also exhibiting substantially reduced diffusion in tissue, thus leading to better spot localization. The ability of these tattoo-ink constituents to induce IRF3 and NFκB inflammatory pathways in cells were evaluated in vitro using J774-DUAL mouse macrophage reporter cells and indicated minimal immunogenicity from these tattoo-ink constituents. Preclinical studies in immunocompetent Balb/c mice using subcutaneous injections indicate extensive diffusion of the commercial ink within the subcutis and significant leakage into the hypodermis. However, our formulations of biomaterial-based tattoo-ink demonstrated high contrast, effective spot localization in the subcutis and no leakage to other tissues, indicating better performance for visualization in vivo. Histopathology analyses on H&E-stained sections revealed lead tattoo-ink candidates with biomaterials chemistry-dependent biocompatibility responses and very minimal immune response in vivo. Additionally, our tattoo-ink formulation was visualized under clinical MR-imaging system which enhances its potential use in multimodal imaging capability with non-invasive follow-up procedure. These biomaterial-based tattoo-ink formulations represent a next generation biocompatible endoscopic ink which will have measurable impacts on patient quality of life and procedural outcomes with a clear path to clinical translation.