(517e) Factorial Design Evaluation of Acesulfame K, Irgasan and Caffeine Removal from Water Using Lignocellulosic Food Residues As Bioadsorptive Materials in Packed Bed Filtration
AIChE Annual Meeting
2022
2022 Annual Meeting
Process Development Division
Materials and Processes for Water Purification and Desalination II
Wednesday, November 16, 2022 - 2:10pm to 2:35pm
Many substances can pollute water, but there are some that are rarely mentioned. Even these substances are usually ignored in a water quality analysis. Among these, emerging pollutants such as highly toxic organic substances, both for the environment and for humans, stand out [3]. Indeed, an investigation conducted in Ecuador confirms that the rivers that collect polluted waters in Quito and flow into Esmeraldas, to the Pacific Ocean, with common organic substances such as caffeine, have been seriously affected [4]. In this research, we will call those substances as emerging organic pollutants (EOPs). Caffeine (CAF), acesulfame K (ACSK), and Irgasan (IRG) are some of these organic substances commonly found in many households that lead to water pollution. Due to this, the need for their removal from water is urgent [5].
Wastewater recovery has traditionally taken place through traditional water treatment plants. These include highly effective processes to eliminate or reduce pollution indicators such as turbidity and total fecal coliforms, among others. However, these plants do not specialize exclusively in depolluting the so-called EOPs. For this reason, very few conventional treatments include a post-treatment to eliminate what has been identified as a problem. These technologies are being studied more and more. Some very effective ones are already known: for example, adsorption with different adsorbent materials especially activated carbon, or filtration with packed beds. More advanced processes, such as advanced oxidation, are also under study [6].
A widely used technology that requires relatively little raw material for use as a depollutant is the packed bed filtration, which uses columns filled with a material with sufficient adsorbent capacity for one or more specific pollutants [7]. Different bed configurations, such as the activated carbon filter [8], have been used according to the pollutant to be removed. Nowadays, since there are organic matter wastes coming mainly from the food industry, biofilters are being increasingly evaluated for their performance, with lignocellulosic residual biomass and biochar being the most investigated materials.
Biomass, derived from plants such as Moringa oleifera Lam. (MO) and Mangifera indica L. (MAN) seeds, promises to be good candidates for use as a packed bed for filters, especially due to its fibrous structure and full of empty spaces where pollutants can adhere [9]. The cell walls allow the formation of a structure capable of physically retaining molecules and ions, adsorbing them, and without presenting any chemical interaction. For example, the irregular morphology of the moringa seed husk and its chemical composition (low amount of protein and high carbohydrate content) allows heavy metal ions to adhere to this substrate easily [10]. Additionally, pretreatments to these lignocellulosic materials are effective in activating more related areas of the substrate where different adsorption processes can occur [9]. Rice husk biochar (BIOCHAR) is a good example of pretreatment transformation for lignocellulosic materials. Given the advantages of using these materials, the main objective of this research is to contrast the removal efficiency of the different materials with each other and with a granular activated carbon (GAC) control, studying this capacity in packed bed filters on emerging pollutants present in polluted water.
In this study, a 4x3x2 factorial design was performed. The factors studied were lignocellulosic residual biomass, emerging organic pollutants, and the initial concentration of these pollutants. The biomass factor consisted of four levels, corresponding to the beds used: MAN, MO, BIOCHAR, and GAC. Three levels were used in the emerging contaminant factor: CAF, ACSK, and IRG. The initial concentrations of these were the third factor to be studied: 5 mg L-1 and 10 mg L-1. All experiments were performed in triplicate (R1, R2, R3), with 72 experimental runs in total. Packed-bed filters consist of a 1-cm diameter borosilicate glass tube, where bed materials were introduced. Each material was packed inside the column filling a height of 30 cm. Filtration beds were not compacted to avoid a lack of internal pathways where water could flow. Sample solutions of the pollutants were injected into the packed bed filters one by one using the pump. Solutions were let flow inside the dry filter, and 5 mL aliquots of the effluent were taken in an interval of two hours of the experimental run. Aliquots were immediately analyzed using the UV-VIS spectrophotometer, and the data were saved for later processing. Every adsorption experiment was run in triplicate. CAF absorbance was measured at 272 nm, ACSK at 226 nm, and IRG at 282 nm. Characterization of the biomaterials was performed by FTIR spectroscopy before and after the adsorption experiments.
FTIR characterization showed active sites of the biomaterials where EOPs can be attached to the surface. In addition, in all acquired FTIR spectra, it can be observed that there was no or very little difference between the bed material before use (MAN, MO, BIOCHAR, and GAC) and the material after the filtration process (CAF, ACSK, and IRG). The peaks of the beds were defined a little better after use, especially in O-H vibrational regions, mainly due to contact with water and pollutants. Since there are no differences in the surface structure of all the beds, it is presumed that the process carried out is precisely adsorption since it only involves physical forces that do not change the molecular structure of the surface of the materials, and therefore there are no chemical bonds that are formed in the process, something that researchers already noticed in a study [11].
In general, it was found that for an initial concentration of 5 mg L-1, the maximum removal was achieved with MAN after two hours of the experimental run. The maximum removal for CAF was 45.76% ± 2.19%. For ACSK, it was 76.83% ± 0.60%, and for IRG, it was 92.69% ± 3.03%. When the initial concentration of contaminants increased to 10 mg L-1, the removal capacity in percentage decreased by about 40%. With respect to control (GAC), this had comparably higher efficiency than all the materials studied. However, similar to what happened with MO, the removal capacity was greater at the beginning of the experimental runs, and after the first 20 minutes, this capacity was reduced, probably due to the saturation of the biomaterial.
Finally, the results reflect that these residues can be used in this type of filters, and it is suggested the investigation of the combination of these materials for the manufacturing of composite filters. This is not only a viable solution to water recovery, but also consists of the reuse and use of lignocellulosic waste, a great economic saving as an alternative to synthetic and expensive mater. Food residues are renewable raw materials that can be used as adsorbent materials in multilayer bed packed columns which are simple, easy to handle and can be a solution for people in rural areas to have clean and safe drinking water while creating job opportunities.
References
[1] C. FN, M. MF, Journal of Ecosystem & Ecography 2017, 07, DOI 10.4172/2157-7625.1000225.
[2] United Nations, The 2030 Agenda and the Sustainable Development Goals: An Opportunity for Latin America and the Caribbean, United Nations, Santiago, 2018.
[3] C. Feng, L. Yu, Y. Xiao, C. An, Journal of Chemistry 2020, 2020, 1.
[4] A. Voloshenko-Rossin, G. Gasser, K. Cohen, J. Gun, L. Cumbal-Flores, W. Parra-Morales, F. Sarabia, F. Ojeda, O. Lev, Environ. Sci.: Processes Impacts 2015, 17, 41.
[5] C. A. Marasco Júnior, N. D. C. Luchiari, P. C. F. Lima Gomes, Eclética Química Journal 2019, 44, 11.
[6] R. Miera, N. Shaikh, K. Artyushkova, A.-M. S. Ali, C. Santoro, B. M. Thomson, K. J. Howe, J. M. Cerrato, Environmental Science: Water Research & Technology 2021, 7, 134.
[7] J. O. Ighalo, A. G. Adeniyi, E. O. Oke, L. T. Adewoye, F. O. Motolani, European Journal of Sustainable Development Research 2020, 4, em0132.
[8] R. P. Rajathi, International Journal for Research in Applied Science and Engineering Technology 2018, 6, 2466.
[9] L. M. Orejuela-Escobar, A. C. Landázuri, B. Goodell, Journal of Bioresources and Bioproducts 2021, 6, 83.
[10] S. H. Guzmán-Maldonado, M. J. López-Manzano, T. J. Madera-Santana, C. A. Núñez-Colín, C. P. Grijalva-Verdugo, A. G. Villa-Lerma, J. R. Rodríguez-Núñez, Agronomía Colombiana 2020, 38, 287.
[11] W. S. Alencar, E. Acayanka, E. C. Lima, B. Royer, F. E. de Souza, J. Lameira, C. N. Alves, Chemical Engineering Journal 2012, 209, 577.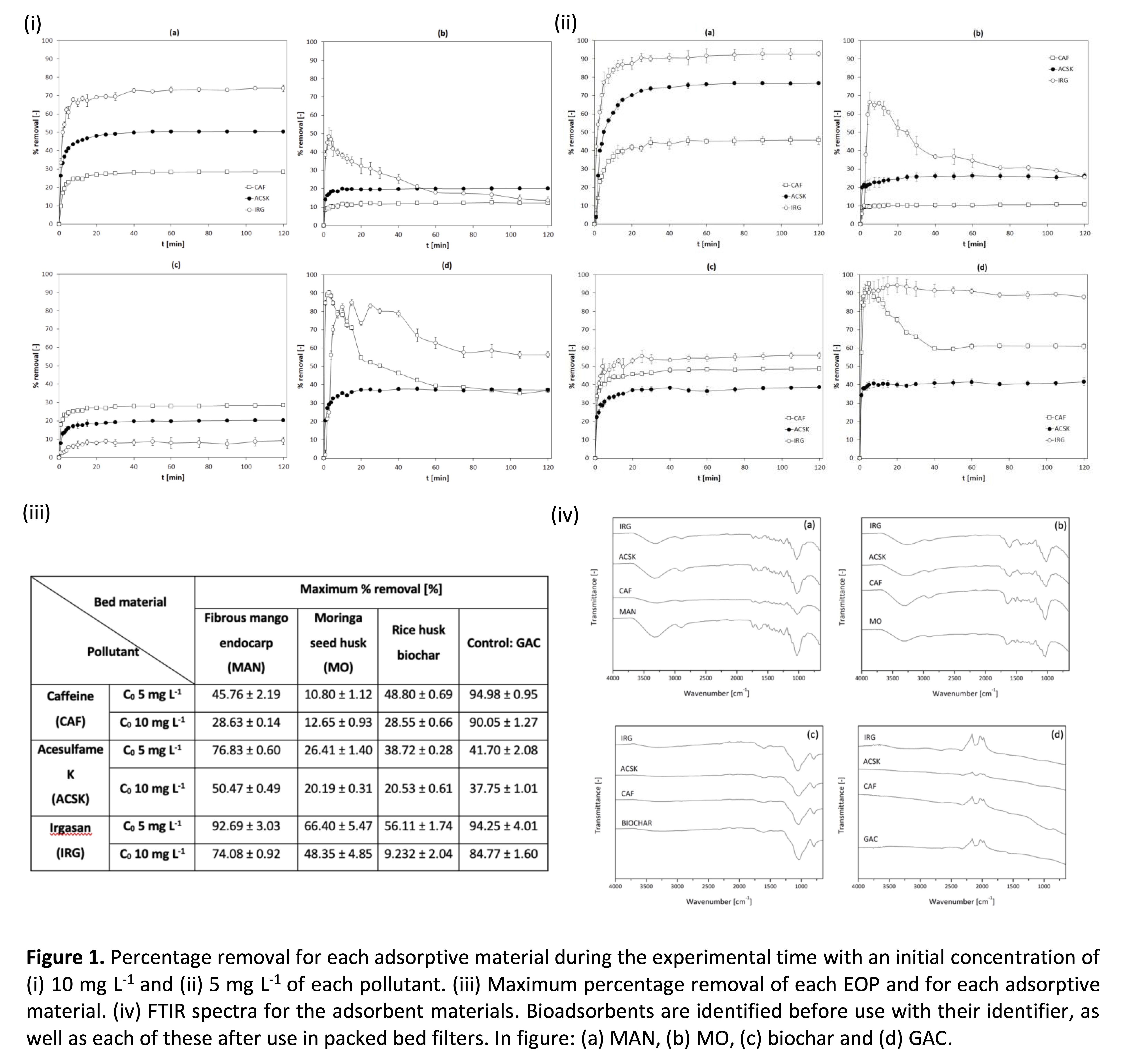
Checkout
This paper has an Extended Abstract file available; you must purchase the conference proceedings to access it.
Do you already own this?
Log In for instructions on accessing this content.
Pricing
Individuals
| AIChE Pro Members | $150.00 |
| AIChE Emeritus Members | $105.00 |
| AIChE Graduate Student Members | Free |
| AIChE Undergraduate Student Members | Free |
| AIChE Explorer Members | $225.00 |
| Non-Members | $225.00 |
