(467g) Monolithic Silica Microchannels Enable Thin Layer Chromatography Analysis of Single Cells
AIChE Annual Meeting
2022
2022 Annual Meeting
Separations Division
Next Generation Biomolecules and Bioprocesses
Wednesday, November 16, 2022 - 10:00am to 10:20am
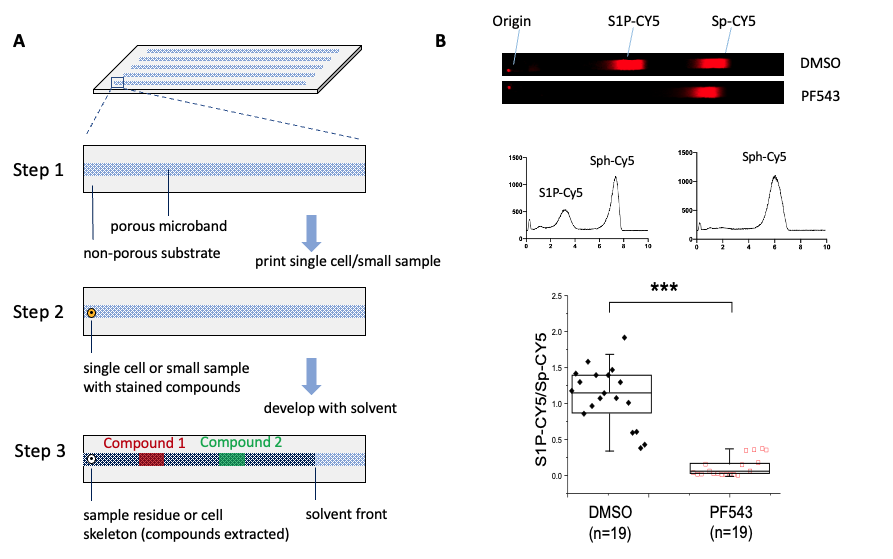
Materials and Methods. The pTLC chip was fabricated by combining sol-gel chemistry and microfabrication technology. pTLC consists of a single or an array of microscale bands made from highly porous monolithic silica designed to accept picoliter-scale volume samples (Figure A). The width and depth of each band was 80 µm and 12 µm, respectively. Droplets of model fluorescent compounds with a volume of 34 pL were spotted on the microband using an ink-jet microdispenser (Microfab Technologies) and then successfully separated upon exposure of the channel inlet to 1-butanol:water:acetic acid (18:4:4 vol:vol:vol). To demonstrate single-cell analysis, K562 cells were loaded with sphingosine (2-amino-4-trans-octadecene-1,3-diol)-CY5 and single cells were then spotted on separate microbands. The cellular metabolites were extracted from the individual cells and separated using pTLC.
Results and Discussion. Model lipids, 18:1 PE CF (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein)) and Texas Red DHPE(1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine), were successfully separated using in as little as 3 min with a separation distance as short as 360 µm. Since Texas Red DHPE has more charged groups (two tertiary amines, one sulfonate) than 18:1 PE CF (one carbonate), Texas Red DHPE had more interaction with the surface charge of the silica resulting in a slower migration rate relative to that of 18:1 PE CF.
To further demonstrate the power of pTLC, single cells were assayed for enzymatic production of sphingosine 1-phosphate (S1P) from sphingosine (Sph). S1P is a metabolic product of Sph formed by the action of sphingosine kinase (SK) as part of an important signal transduction pathway within cells. Analysis of S1P and Sph of biological samples is useful in revealing lipid signaling activity in many diseases including cancer, autoimmune and neurological diseases. S1P supports cell proliferation in tumors and thus fuels their malignant behavior so that SK is often mutated, overexpressed or otherwise upregulated in tumor cells.
To assess the separation and detection of Sph and S1P, fluorescein-labeled Sph (SphF) and S1P (S1PF) in solution was spotted at the inlet of the channels of a pTLC plate followed by separation. The average migration speed of the analytes was 170 µm for SphF and 36 µm for S1PF over the time of 1 min. The negative phosphate group of S1PF hinders its migration due to its interaction with silica. The above results demonstrate that pTLC is capable of separating micro-spotted organic compounds. One unique advantage of pTLC was that the migration was confined along the microbands without lateral diffusion. Other derivatized lipids were suitable for separation and assay by pTLC, including Bodipy FL PIP2 (Phosphatidylinositol 4,5-bisphosphate) and PIP3 (Phosphatidylinositol 3,4,5-trisphosphate), PC-CY5 and PE-CY5. Using PC-CY5 (1,2-dioleoyl-sn-glycero-3-phosphocholine-N-(Cyanine 5)) and PE-CY5 (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(Cyanine 5)), the detection limit of analytes of pTLC was 3×10-19 mole, which is significantly better than commercial high-performance TLC (HPTLC) plates.
To demonstrate the ability to assay single-cell SK activity, a single cell loaded with Sph-CY5 was spotted on a pTLC microband. The pTLC plate was developed in 1-butanol:1-propanol:water:triethylamine (18:2:4:1, vol:vol:vol:vol) and the fluorescence of CY5 measured within the channel. Two bands composed of Sph-CY5 and S1P-CY5 eluted from the cell, migrated along the microband, and were separated in 4 min (resolution = 1.8). Quantitative analysis of the pTLC chromatogram at 11 min showed that the S1P-CY5 peak area was 33.8% and Sph-CY5 peak area was 66.2% of the total CY5 fluorescence measured. From these data, this particular cell was shown to convert 33.8% of the loaded Sph-CY5 to S1P CY5 in 30 min via the action of sphingosine kinase. To confirm that the conversion of Sp-CY5 to S1P-CY5 was likely due to SK, cells were analyzed after incubation with the SK inhibitor PF543 (Figure B). As expected, incubation of cells with the inhibitor abrogated the S1P-CY5 peak. This result demonstrated that pTLC can be used for analyzing biologically important compounds at the single-cell level.
Conclusion. We have demonstrated a novel technology for single-cell analysis and picoliter-scale specimens. We have demonstrated the use of pTLC for the analysis of sphingolipid signaling in single cells. We envision pTLC will have potential applications in many areas where miniature specimens and high-throughput parallel analyses are needed, such as diagnosis of diseases, cell heterogeneity analysis, monitoring droplet-based reactions, detecting miniature environmental, food, forensic and pharmaceutical samples.
Reference:
- Proctor, A.; Allbritton, N. L., "Fix and assay": separating in-cellulo sphingolipid reactions from analytical assay in time and space using an aldehyde-based fixative. Analyst 2019, 144 (3), 961-971.
- Nakanishi, K.; Shikata, H.; Ishizuka, N.; Koheiya, N.; Soga, N., Tailoring mesopores in monolithic macroporous silica for HPLC. HRC-J. High Resolut. Chromatogr. 2000, 23 (1), 106-110.
- Jiang, Y.; Kang, X.; Gao, D.; He, A.; Guo, R.; Fan, X.; Zhai, Y.; Xia, J.; Xu, Y.; Noda, I.; Wu, J., Finding a suitable separation condition for TLC/FTIR analysis by using multiple-narrow-band TLC technique. RSC Advances 2015, 5 (28), 21544-21549.
- Castano-Alvarez, M.; Ayuso, D. F. P.; Granda, M. G.; Fernandez-Abedul, M. T.; Garcia, J. R.; Costa-Garcia, A., Critical points in the fabrication of microfluidic devices on glass substrates. Sens. Actuator B-Chem. 2008, 130 (1), 436-448.
- Fujima, T.; Futakuchi, E.; Tomita, T.; Orai, Y.; Sunaoshi, T., Hierarchical Nanoporous Glass with Antireflectivity and Superhydrophilicity by One-Pot Etching. Langmuir 2014, 30 (48), 14494-14497.
- Hara, T.; Desmet, G.; Baron, G. V.; Minakuchi, H.; Eeltink, S., Effect of polyethylene glycol on pore structure and separation efficiency of silica-based monolithic capillary columns. J. Chromatogr. A 2016, 1442, 42-52.