(42f) Effect of Water and CO on the NOx Operating Cycle in Pd/CHA Passive NOx Adsorbers
AIChE Annual Meeting
2022
2022 Annual Meeting
Catalysis and Reaction Engineering Division
Environmental Catalysis I: Applied Catalysis for Emissions Control
Monday, November 14, 2022 - 9:20am to 9:40am
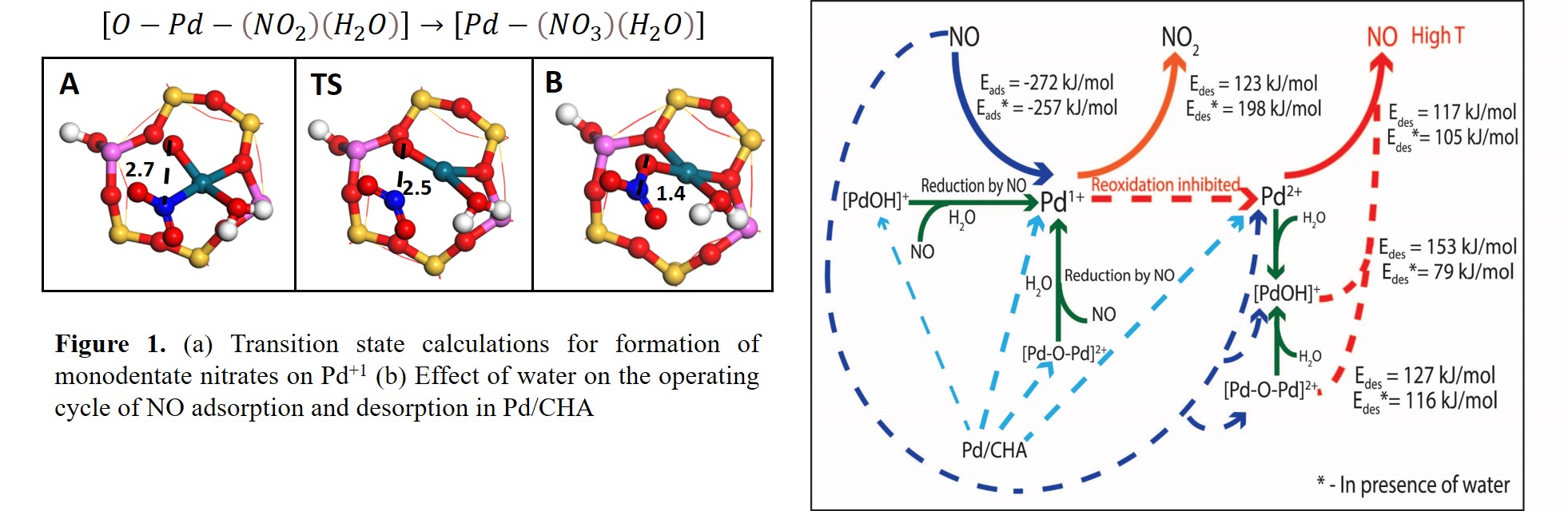
H2O and CO affect the NOx adsorption/desorption characteristics of Pd/CHA passive NOx adsorbers. However, the presence of various Pd sites (Pd2+, Pd1+, [PdOH]+, and [Pd-O-Pd]2+) makes it challenging to decipher their role on the NOx adsorption-desorption cycle. DFT calculations are performed in this work to understand the impact of H2O and CO on NO interaction with various Pd sites, and explain the mechanisms of NO adsorption, oxidation, and desorption in their presence. Calculations show that while H2O has a negligible effect on the NO adsorption energetics for various Pd sites (Pd2+, Pd1+, and [Pd-O-Pd]2+), it significantly weakens the NO binding on [PdOH]+. However, the presence of CO on a [PdOH]+ site with pre-adsorbed H2O strengthens NO binding. Even though the presence of H2O facilitates the reduction of [PdOH]+ and [Pd-O-Pd]2+ by NO, it negatively impacts the desorption of NO2 (formed by NO oxidation) from the Pd1+ sites. In addition, CO is shown to be a better reductant as compared to NO when both CO and NO are co-adsorbed on [Pd-O-Pd]2+, thus resulting in the formation of Pd1+. The Pd1+ sites can reoxidize to PdII species under an oxidizing atmosphere, such that the NO desorption is facilitated. However, in the presence of H2O, NO oxidation is favored over Pd1+ reoxidation, thus resulting in the formation of monodentate nitrates (Figure 1(a)). The monodentate nitrates transform to the more stable bidentate nitrates upon water desorption. This finding, along with the higher NO2 desorption energy in the presence of H2O can explain the increase in the NOx desorption temperature in the presence of H2O. The proposed effect of H2O on the NOx operating cycle is depicted in Figure 1(b). In the full paper, the inferences obtained on the effect of H2O and CO will be used to explain the reported experimental data.